Abstract
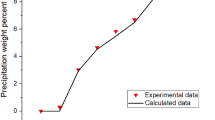
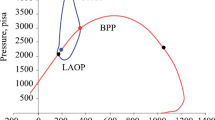
Asphaltene deposition causes serious problems in the oil industry and reduces oil recovery. Deposition happens as a consequence of asphaltene precipitation which is a process as a result of a change in thermodynamic stability. Thus, prediction and preventing of the precipitation condition are the first step of preventing asphaltene precipitation and deposition. In this study, a thermodynamic model for asphaltene precipitation has been developed using Peng–Robinson (PR), Soave–Redlich–Kwong (SRK), and a new modification on SRK [modified-SRK equations of state (EOS)]. To modify EOS for non-pure sample (oil sample), van der Waals mixing rule with three types of combining rule containing conventional, Margules, and van Laar type was used. In addition, to verify the derived model, the experiments were conducted on a live oil sample to investigate the effect of pressure reduction and gas injection [nitrogen (0.1, 0.2, and 0.4 mol fraction) and first stage gas (0.2, 0.4 and 0.6 mol fraction)] on asphaltene precipitation. The results show that at low pressures (pressures below 5000 psia), nitrogen is not soluble in oil and the injection of nitrogen reduces asphaltene precipitation because of the liberation of the light component from crude oil; however, increasing the pressure (pressures above 6000 psia) increases the solubility of nitrogen and increases the asphaltene precipitation. For the first stage gas injection, asphaltene precipitation increases because of its high solubility in crude oil at any pressure. The amount of asphaltene precipitation due to first stage gas injection is higher than nitrogen injection except at nitrogen concentration and pressures near the bubble point (pressure of 7000 psia and nitrogen injection of 0.1 mol fraction). According to the modeling results, van Laar type combining rule in conjugated with modified-SRK-EOS predicts the amount of asphaltene precipitation very well at all situations of pressures and different gas injections, and has the least deviation from experimental data rather than the other two types of combining rules; and using mentioned combining formula, the RMSE value decreases to about 50% of the conventional combining rule. It is because of the accurate and distinct interaction parameters of each pair of components in van Laar equation.











Similar content being viewed by others
Abbreviations
- APCI:
-
Atmospheric pressure chemical ionization
- a :
-
Attractive-energy parameter of EOS
- a mixture :
-
Attractive-energy parameter of EOS for mixtures
- a i :
-
Attractive-energy parameter of component “i”
- a ij :
-
Cross term of parameter “a” for pair of components “i” and “j”
- b :
-
Co-volume parameter of EOS
- b mixture :
-
Co-volume parameter of EOS for mixtures
- α, ψ, Ω:
-
EOS parameters
- Ω:
-
Acentric factor
- Cal.datai :
-
Calculated parameter with a model at Exp.datai condition
- Exp.datai :
-
ith experimental data
- EOS:
-
Equation/s of state
- FCS:
-
Fluorescence correlation spectroscopy
- FDFI:
-
Fluorescence depolarization field ionization
- FI:
-
Field ionization
- FT-ICR:
-
Fourier transform ion cyclotron resonance
- \(f_{\text{As}}^{\text{oil}}\) :
-
Fugacity of asphaltene in residue oil
- \(f_{\text{As}}^{\text{pure}}\) :
-
Fugacity of precipitated asphaltene
- f i :
-
Fugacity of component “i” in reservoir oil
- \(f_{i}^{\text{gas}}\) :
-
Fugacity of component “i” in gas phase
- \(f_{i}^{\text{oil}}\) :
-
Fugacity of component “i” in residue oil
- \(f_{i}^{\text{pure}}\) :
-
Fugacity of component “i” in pure state
- K ij :
-
Interaction parameter of component “i” with “j”
- GOR:
-
Gas oil ratio
- GPC:
-
Gel permeation chromatography
- LD:
-
Laser desorption
- LDI:
-
Laser desorption ionization
- LOP:
-
Lower onset pressure
- MS:
-
Mass spectroscopy
- MW:
-
Molecular weight
- N :
-
Number of experimental data
- P :
-
Pressure
- P b :
-
Bubble point pressure
- P c :
-
Critical pressure
- PR:
-
Peng–Robinson
- P R :
-
Reservoir pressure
- P r :
-
Reduced pressure
- R :
-
Universal gas constant
- RMSE:
-
Root-mean-square error
- SALDI:
-
Surface-assisted laser desorption/ionization
- SEC:
-
Size exclusion chromatography
- SRK:
-
Soave–Redlich–Kwong
- T :
-
Temperature
- T c :
-
Critical temperature
- T R :
-
Reservoir temperature
- T r :
-
Reduced temperature
- θ 1, θ 2, θ 3 :
-
Adjustable parameters of Kij for PR-EOS
- ξ, δ :
-
Parameters of Kij for SRK-EOS
- TR-FD:
-
Time-resolved fluorescence depolarization
- UOP:
-
Upper onset pressure
- wt%:
-
Weight percent
- \(x_{\text{As}}\) :
-
Mole fraction of asphaltene in residue oil
- \(x_{i}\) :
-
Mole fraction of component “i” in residue oil
- \(y_{i}\) :
-
Mole fraction of component “i” in gas phase
- \(y_{i}^{\text{b}}\) :
-
Mole fraction of component “i” in gas phase at bubble point pressure
- \(Z_{i}\) :
-
Mole fraction of component “i” in reservoir oil
References
Abedini R, Abedini A (2011) Development of an artificial neural network algorithm for the prediction of asphaltene precipitation. Pet Sci Technol 29:1565–1577
Abedini A, Ashoori S, Torabi F (2011) Reversibility of asphaltene precipitation in porous and non-porous media. Fluid Phase Equilib 308:129–134
Acevedo S, Gutierrez L, Negrin G, Pereira J, Mendez B, Delolme F, Dessalces G, Broseta D (2005) Molecular weight of petroleum asphaltenes: a comparison between mass spectrometry and vapor pressure osmometry. Energy Fuels 19(4):1548–1560
Ahmadi MA (2012) Neural network based unified particle swarm optimization for prediction of asphaltene precipitation. Fluid Phase Equilib 314:46–51
Akbarzadeh K, Hammami A, Kharrat A, Zhang D, Allenson S, Creek J, Kabir S, Jamaluddin A, Marshall A, Rodgers R (2007) Asphaltenes-problematic but rich in potential. Oilfield Rev 19(2):22–43
Andrews AB, Guerra R, Sen PN, Mullins OC (2006) Diffusivity of asphaltene molecules by fluorescence correlations spectroscopy. J Phys Chem 110:8095
Arya A, Liang X, Von Solms N, Kontogeorgis GM (2016) Modeling of asphaltene onset precipitation conditions with cubic plus association (CPA) and perturbed chain statistical associating fluid theory (PC-SAFT) equations of state. Energy Fuels 30(8):6835–6852
Arya A, Liang X, von Solms N, Kontogeorgis GM (2017) Prediction of gas injection effect on asphaltene precipitation onset using the cubic and cubic-plusassociation equations of state. Energy Fuels 31(3):3313–3328
Ashoori S, Balavi A (2014) An investigation of asphaltene precipitation during natural production and the CO2 injection process. Pet Sci Technol 32(11):1283–1290
Ashoori S, Abedini A, Abedini R, Qorbani Nasheghi Kh (2010) Comparison of scaling equation with neural network model for prediction of asphaltene precipitation. J Pet Sci Eng 72:186–194
Aske N, Kallevik H, Johnsen EE, Sjoblom J (2002) Asphaltene aggregation from crude oils and model systems studied by high-pressure NIR spectroscopy. Energy Fuels 16:1287–1295
Badre S, Carla C, Norinaga K, Gustavson G, Mullins OC (2006) Molecular size and weight of asphaltene and asphaltene solubility fractions from coals, crude oils and bitumen. Fuel 85:1–11
Barrera DM, Ortiz DP, Yarranton HW (2013) Molecular weight and density distribution of asphaltene from crude oils. Energy Fuels 27:2472–2487
Bouhadda Y, Bormann D, Sheu E, Bendedouch D, Krallafa A, Daaou M (2007) Characterization of Algerian Hassi-Messaoud asphaltene structure using Raman-spectrometry and X-ray diffraction. Fuel 86:1855–1864
Buenrostro-Gonzalez E, Lira-Galeana C, Gil-Villegas A, Wu J (2004) Asphaltene precipitation in crude oils: theory and experiments. AIChE J 50:2552–2570
Cunico RI, Sheu EY, Mullins OC (2004) Molecular weight measurement of UG8 asphaltene using APCI mass spectroscopy. Pet Sci Technol 22:787–798
De Boer RB, Leerlooyer K, Eigner MR, Van Bergen AR (1992) SPE 24987 screening of crude oils for asphalt precipitation: theory, practice, and the selection of inhibitors. In: European petroleum conference, pp. 259
Duda Y, Lira-Galeana C (2006) Thermodynamics of asphaltene structure and aggregation. Fluid Phase Equilib 241:257–267
Fateen SEK, Khalil MM, Elnabawy AO (2013) Semi-empirical correlation for binary interaction parameters of the Peng–Robinson equation of state with the van der Waals mixing rules for the prediction of high-pressure vapor–liquid equilibrium. J Adv Res 4:137–145
Hajizadeh N, Moradi GR, Ashoori S (2020) Modified SRK equation of state for modeling asphaltene precipitation. J Chem React Eng 18(3)
Hemmati-Sarapardeh A, AliPour-Yeganeh-Marand R, Naseri A, Safiabadi A, Gharagheizi F, Ilani-Kashkouli P, Mohammadi AH (2013) Asphaltene precipitation due to natural depletion of reservoir: determination using a SARA fraction based intelligent model. Fluid Phase Equilib 354:177–184
Hustad OS, Jia N, Pedersen KS, Memon A, Leekumjorn S (2014) High-pressure data and modeling results for phase behavior and asphaltene onsets of Gulf of Mexico oil mixed with nitrogen. SPE Reservoir Eval Eng 17(03):384–395
Hutado P, Hortal AR, Martinez-Haya B (2007) MALDI detection of carbonaceous compounds in ionic liquid matrices. Mass Spectrom 21:3161–3164
Ikeda MK, Schaefer LA (2011) Examination the effect of binary interaction parameters on VLE modeling using cubic equations of state. Fluid Phase Equilib 305:233–237
Jafari Behbahani T, Ghotbi C, Taghikhani V, Shahrabadi A (2011) Experimental investigation and thermodynamic modeling of asphaltene precipitation. Sci Iran 18(6):1384–1390
Jamaluddin AKM, Creek J, Kabir CS, Mcfadden JD, D’Cruz D, Manakalathil J, Joshi N, Ross B (2002) Laboratory techniques to measure thermodynamic asphaltene instability. J Can Pet Technol 41(07)
Jaubert JN, Privat R (2010) Relationship between the binary interaction parameters (kij) of the Peng–Robinson and those of the Soave–Redlich–Kwong equations of state: application to the definition of the PR2SRK model. Fluid Phase Equilib 295:26–37
Kontogeorgis GM, Folas GK (2010) Thermodynamic models for industrial applications. Wiley, New York
Merdrignac I, Desmazieres B, Terrier P, Delobel A, Laprevote O (2004) Analysis of raw and hydrotreated asphaltenes using off-line and on-line SEC/MS coupling. In: Proceedings of the Heavy Organic Deposition, Los Cabos, Baja California, Mexico
Miller JT, Fisher RB, Thiyagarajan P, Winans RE, Hunt JE (1998) Subfractionation and characterization of Mayan asphaltene. Energy Fuels 12:1290–1298
Mohammadi AH, Richon D (2007) A monodisperse thermodynamic model for estimating asphaltene precipitation. AIChE J 53:2940–2947
Mullins OC, Martínez-Haya B, Marshall AG (2008) Contrasting perspective on asphaltene molecular weight. This comment vs the overview of A.A. Herod, K.D. Energy Fuels 22(3):1765–1773
Mullins OC, Sabbah H, Eyssautier J, Pomerantz AE, Barré L, Andrews AB, Ruiz-Morales Y, Mostowfi F, McFarlane R, Goual L, Lepkowicz R (2012) Advances in asphaltene science and the Yen–Mullins model. Energy Fuels 26(7):3986–4003
Orangi HS, Modarress H, Fazlali A, Namazi MH (2006) Phase behavior of binary mixture of asphaltene + solvent and ternary mixture of asphaltene + solvent + precipitant. Fluid Phase Equilib 245:117–124
Pazuki GR, Nikookar M, Omidkhah MR (2007) Application of a new cubic equation of state to computation of phase behavior of fluids and asphaltene precipitation in crude oil. Fluid Phase Equilib 254:42–48
Peramanu S, Singh C, Agrawala M, Yarranton HW (2001) Investigation on the reversibility of asphaltene precipitation. Energy Fuels 15:910–917
Pomerantz AE, Wu Q, Mullins OC, Zare RN (2015) Laser-based mass spectrometric of asphaltene molecular weight, molecular architecture and nanoaggregate number. Energy Fuels 29:2833–2842
Qian K, Edwards KE, Siskin M, Olmstead WN, Mennito AS, Dechart GJ, Hoosain NE (2007) Desorption and ionization of heavy petroleum molecules and measurement of molecular weight distribution. Energy Fuels 21:1042–1047
Rastgoo A, Kharrat R (2017) Investigation of asphaltene deposition and precipitation in production tubing. Int J Clean Coal Energy 6:14–29
Rodgers RP, Marshall AG (2007) Petroleomics: advanced characterization of petroleum-derived materials by Fourier transform ion cyclotron resonance mass spectrometry (FT-ICR MS). In: Asphaltenes, heavy oils, and petroleomics: Springer; p. 63–93
Sabbagh O, Akbarzadeh K, Badamchi-Zadeh A, Svrcek WY, Yarranton HW (2006) Applying the PR-EOS to asphaltene precipitation from n-alkane diluted heavy oils and bitumens. Energy Fuels 20:625–634
Shirani B, Nikazar M, Naseri A, Mousavi-Dehghani SA (2012) Modeling of asphaltene precipitation utilizing association equation of state. Fuel 93:59–66
Soleymanzadeh A, Yousefi M, Kord S, Mohammadzadeh O (2019) A review on methods of determining onset of asphaltene precipitation. J Pet Explor Prod Technol 9(2):1375–1396
Subramanian S, Simon S, Sjoblom J (2016) Asphaltene precipitation models: a review. Dispers Sci Technol 37:1027–1049
Thomas F, Bennion D, Bennion D, Hunter B (1992) Experimental and theoretical studies of solids precipitation from reservoir fluid. J Can Pet Technol 31(01)
Wang P, Zhao F, Hou J, Lu G, Zhang M, Wang Zh (2018) Comparative analysis of CO2, N2 and gas mixture injection on asphaltene deposition pressure in reservoir conditions. Energies 11(9):2483
Zeinali Hasanvand M, Montazeri M, Salehzadeh M, Amiri M, Fathinasab M (2018) A literature review of asphaltene entity, precipitation and deposition: introducing recent models of deposition in the well column. J Oil Gas Petrochem Sci 1(3):83–89
Zendehboudi S, Ahmadi MA, Mohammadzadeh O, Bahadori A, Chatzis I (2013) Thermodynamic investigation of asphaltene precipitation during primary oil production: laboratory and smart technique. Ind Eng Chem Res 52(17):6009–6031
Zendehboudi S, Shafiei A, Bahadori AR, James LA, Elkamel A, Lohi A (2014) Asphaltene precipitation and deposition in oil reservoirs–technical aspects, experimental and hybrid neural network predictive tools. Chem Eng Res Des 92:857–875
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendices
Appendix 1: Asphaltene molecular weight
Asphaltene is a self-association molecule that makes very hard to measuring the accurate molecular weight of it. However, there are many works done for measuring the molecular weight of asphaltene. The results of several important works done on this issue with different experimental methods are shown in Table 9 and Fig. 12.
Abundance of asphaltene molecular weight according to Table 9
Appendix 2: Binary interaction parameters
Equations of state need some binary interaction coefficients to be applicable for mixtures. In this section, binary interaction parameters for different combining rules and different EOS are presented.
-
1.
One-binary interaction parameter (conventional combining rule).
In this type of combining rule, one-binary interaction parameter is used for each pair of components (i.e., Kij = Kji).
Binary interaction coefficients for common pairs of material containing light hydrocarbons for conventional combining rule are available in the literatures (Arya et al. 2017; Hustad et al. 2014). Binary interaction coefficients for asphaltene with other hydrocarbons are calculated according to experimental data available for upper onset pressure of reservoir oil (Hajizadeh et al. 2020) and are presented in Table 10.
Table 10 Binary interaction parameter (Kij) -
2.
Two-binary interaction parameters (Margules type and van Laar type combining rules).
In this type of combining rules, two-binary interaction parameters are used for each pair of components (i.e., Kij ≠ Kji). For this type of combining rule, there is a semi-empirical correlation for binary interaction parameter as Eq. 16 (Fateen et al. 2013):
$$K_{ij} = 1 - \frac{1}{2}\frac{{b_{j} }}{{b_{i} }} \sqrt {\frac{{a_{i} }}{{a_{j} }}} - \frac{1}{2}\frac{{b_{i} }}{{b_{j} }} \sqrt {\frac{{a_{j} }}{{a_{i} }}} + \frac{1}{2}\frac{{b_{j} RT}}{{\sqrt {a_{i} a_{j} } }} \frac{{\theta_{1} }}{{T_{{r_{i} }}^{{\theta_{2} }} P_{{r_{i} }}^{{\theta_{3} }} }}.$$(16)This equation is applicable for PR-EOS with adjustable parameters θ1, θ2, and θ3 which, for some pair of species, are presented by Fateen et al. (2013) and for other species are adjusted in this study. These parameters are listed in Table 11.
Table 11 θ1, θ2, and θ3 for calculation of binary interaction parameter (Kij) of PR-EOS (the parameters in the left side of diagonal line are for Margules and in the right side are for van Laar)
For calculation of binary interaction parameters for SRK-EOS and modified-SRK-EOS, we used the relation between the binary interaction parameter of PR- and SRK-EOS according to Eq. 17 (Jaubert and Privat 2010):
The difference between binary interaction of SRK and modified-SRK-EOS is the amount of “\(\delta_{\text{Asp}}\)” parameter because of difference in “b” parameter of these two EOS, according to Table 1.
Also the amount of “ξ” in Jaubert and Privat (2010), assumed to be constant about 0.807341 for SRK-EOS, we changed it to ξ = 0.794 for modified-SRK-EOS according to Eq. (19).
Rights and permissions
About this article
Cite this article
Hajizadeh, N., Moradi, G. & Ashoori, S. Effect of combining rules on modeling of asphaltene precipitation. Chem. Pap. 75, 2851–2870 (2021). https://doi.org/10.1007/s11696-020-01479-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-020-01479-6