Abstract
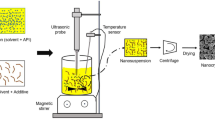
A continuous microfluidic nanoprecipitation process has been investigated to prepare nanosized particles of a poorly water-soluble drug telmisartan (TEL), thereby enhancing its solubility and bioavailability. The present work aims to overcome agglomeration of drug particles by controlling the surface forces between the particles using various polymers like Polyvinylpyrrolidone K-30 (PVP K-30), Polyvinylpyrrolidone K-90 (PVP K-90), Poloxamer 188, Poloxamer 407, and hydroxypropyl methylcellulose (HPMC). The effect of process parameters such as solvent-to-antisolvent ratio, polymer-to-drug ratio, microchannel length, and solvent flow rate on drug particle size and polydispersity index (PDI) has been studied. The drug–polymer interaction investigated through X-ray diffraction (XRD) and Fourier transform infrared (FTIR) analysis revealed a significant reduction in peak intensity for Poloxamer 407 with no drug–polymer interaction. Also, the surface morphology of recrystallised TEL nanoparticles examined using field emission scanning electron microscopy (FESEM) showed clear and nearly uniform shaped particles. Poloxamer 407-based formulation of TEL exhibited minimum drug agglomeration with least particle size 369 nm and PDI value 0.049. The minimum particle size was achieved at solvent-to-antisolvent ratio 1:2, 1:1 polymer-to-drug ratio, microchannel length of 60 cm, and solvent flow rate of 30 mL/h. Thus, the microfluidic technique resulted in the production of TEL nanoparticles with narrow size distribution and useful morphological characteristics.
Graphic abstract











Similar content being viewed by others
Abbreviations
- APIs:
-
Active pharmaceutical ingredients
- BCS:
-
Biopharmaceutical Classification System
- FESEM:
-
Field emission scanning electron microscopy
- FTIR:
-
Fourier transform infrared
- HPMC:
-
Hydroxypropyl methylcellulose
- PDI:
-
Polydispersity index
- PEO:
-
Polyethylene oxide
- PPO:
-
Polypropylene oxide
- PVP K-30:
-
Polyvinylpyrrolidone K-30
- PVP K-90:
-
Polyvinylpyrrolidone K-90
- TEL:
-
Telmisartan
- XRD:
-
X-ray diffraction
References
Bhise S, Mathure D, Patil MV, Patankar RD (2011) Solubility enhancement of antihypertensive agent by solid dispersion technique. Int J Pharm Life Sci 2:970–995
Chae JS et al (2018) Tablet formulation of a polymeric solid dispersion containing amorphous alkalinized telmisartan. AAPS PharmSciTech 19:2990–2999
de Solorzano IO, Uson L, Larrea A, Miana M, Sebastian V, Arruebo M (2016) Continuous synthesis of drug-loaded nanoparticles using microchannel emulsification and numerical modeling: effect of passive mixing. Int J Nanomed 11:3397
Dong Z, Rivas DF, Kuhn S (2019) Acoustophoretic focusing effects on particle synthesis and clogging in microreactors. Lab Chip 19:316–327
Dora CP, Singh SK, Kumar S, Datusalia AK, Deep A (2010) Development and characterization of nanoparticles of glibenclamide by solvent displacement method. Acta Pol Pharm 67:283–290
Feng Q, Sun J, Jiang X (2016) Microfluidics-mediated assembly of functional nanoparticles for cancer-related pharmaceutical applications. Nanoscale 8:12430–12443
Frizon F, de Oliveira Eloy J, Donaduzzi CM, Mitsui ML, Marchetti JM (2013) Dissolution rate enhancement of loratadine in polyvinylpyrrolidone K-30 solid dispersions by solvent methods. Powder Technol 235:532–539
Hu J, Johnston KP, Williams RO III (2004) Nanoparticle engineering processes for enhancing the dissolution rates of poorly water soluble drugs. Drug Dev Ind Pharm 30:233–245
Huang Q-P, Wang J-X, Chen G-Z, Shen Z-G, Chen J-F, Yun J (2008) Micronization of gemfibrozil by reactive precipitation process. Int J Pharm 360:58–64
Kakran M, Sahoo N, Li L, Judeh Z, Wang Y, Chong K, Loh L (2010) Fabrication of drug nanoparticles by evaporative precipitation of nanosuspension. Int J Pharm 383:285–292
Kakran M, Sahoo NG, Tan I-L, Li L (2012) Preparation of nanoparticles of poorly water-soluble antioxidant curcumin by antisolvent precipitation methods. J Nanoparticle Res 14:757
Kaufman JJ et al (2012) Structured spheres generated by an in-fibre fluid instability. Nature 487:463–467
Khan J, Bashir S, Khan MA, Mohammad MA, Isreb M (2018) Fabrication and characterization of dexibuprofen nanocrystals using microchannel fluidic rector. Drug Des Devel Ther 12:2617
Landry V, Riedl B, Blanchet P (2008) Alumina and zirconia acrylate nanocomposites coatings for wood flooring: photocalorimetric characterization. Prog Org Coat 61:76–82
Lee M, Kim S, Ahn C-H, Lee J (2010) Hydrophilic and hydrophobic amino acid copolymers for nano-comminution of poorly soluble drugs. Int J Pharm 384:173–180
Li DX et al (2010) Enhanced oral bioavailability of flurbiprofen by combined use of micelle solution and inclusion compound. Arch Pharmacal Res 33:95–101
Malvern I (2004) Zetasizer nano series user manual. Malvern Instruments Ltd, Worcestershire
Matteucci ME, Hotze MA, Johnston KP, Williams RO (2006) Drug nanoparticles by antisolvent precipitation: mixing energy versus surfactant stabilization. Langmuir 22:8951–8959
Mello Jd, Mello Ad (2004) FocusMicroscale reactors: nanoscale products. Lab Chip 4:11N–15N
Othman R, Vladisavljević GT, Nagy ZK (2015) Preparation of biodegradable polymeric nanoparticles for pharmaceutical applications using glass capillary microfluidics. Chem Eng Sci 137:119–130
Ozaki S, Minamisono T, Yamashita T, Kato T, Kushida I (2012) Supersaturation–nucleation behavior of poorly soluble drugs and its impact on the oral absorption of drugs in thermodynamically high-energy forms. J Pharm Sci 101:214–222
Patil P, Khairnar G, Naik J (2015) Preparation and statistical optimization of Losartan Potassium loaded nanoparticles using Box Behnken factorial design: Microreactor precipitation. Chem Eng Res Des 104:98–109
Sangwai M, Vavia P (2013) Amorphous ternary cyclodextrin nanocomposites of telmisartan for oral drug delivery: improved solubility and reduced pharmacokinetic variability. Int J Pharm 453:423–432
Sangwal K (2007) Additives and crystallization processes: from fundamentals to applications. John Wiley & Sons, Chichester
Sharma C, Desai MA, Patel SR (2018) Ultrasound-assisted anti-solvent crystallization of telmisartan using dimethyl sulfoxide as organic solvent. Cryst Res Technol 53:1800001
Sharma C, Desai MA, Patel SR (2019) Effect of surfactants and polymers on morphology and particle size of telmisartan in ultrasound-assisted anti-solvent crystallization. Chem Pap 73:1685–1694
Sharma C, Desai MA, Patel SR (2020) Anti-solvent sonocrystallization for nano-range particle size of telmisartan through Taguchi and Box-Behnken design. Chem Pap 74:323–331
Shrimal P, Jadeja G, Naik J, Patel S (2019a) Continuous microchannel precipitation to enhance the solubility of telmisartan with poloxamer 407 using Box-Behnken design approach. J Drug Deliv Sci Technol 53:101225
Shrimal P, Jadeja G, Patel S (2019b) A review on novel methodologies for drug nanoparticle preparation: microfluidic approach. Chem Eng Res Des
Tai S, Zhang W, Zhang J, Luo G, Jia Y, Deng M, Ling Y (2016) Facile preparation of UiO-66 nanoparticles with tunable sizes in a continuous flow microreactor and its application in drug delivery. Microporous Mesoporous Mater 220:148–154
Wang Z, Chen JF, Le Y, Shen ZG, Yun J (2007) Preparation of ultrafine beclomethasone dipropionate drug powder by antisolvent precipitation. Ind Eng Chem Res 46:4839–4845
Wang JX, Zhang QX, Zhou Y, Shao L, Chen JF (2010) Microfluidic synthesis of amorphous cefuroxime axetil nanoparticles with size-dependent and enhanced dissolution rate. Chem Eng J 162:844–851
Warren DB, Benameur H, Porter CJ, Pouton CW (2010) Using polymeric precipitation inhibitors to improve the absorption of poorly water-soluble drugs: a mechanistic basis for utility. J Drug Target 18:704–731
Wu K-J, Bohan GMDV, Torrente-Murciano L (2017) Synthesis of narrow sized silver nanoparticles in the absence of capping ligands in helical microreactors. React Chem Eng 2:116–128
Zhang H-X, Wang J-X, Shao L, Chen J-F (2010) Microfluidic fabrication of monodispersed pharmaceutical colloidal spheres of atorvastatin calcium with tunable sizes. Ind Eng Chem Res 49:4156–4161
Zhang X, Chen H, Qian F, Cheng Y (2018) Preparation of itraconazole nanoparticles by anti-solvent precipitation method using a cascaded microfluidic device and an ultrasonic spray drier. Chem Eng J 334:2264–2272
Acknowledgements
The corresponding author would like to acknowledge Science and Engineering Research Board, Department of Science and Technology, Government of India (sanctioned no. EEQ/2018/001001, dated 23-05-2019) for financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author reports there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shrimal, P., Jadeja, G. & Patel, S. Microfluidics nanoprecipitation of telmisartan nanoparticles: effect of process and formulation parameters. Chem. Pap. 75, 205–214 (2021). https://doi.org/10.1007/s11696-020-01289-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-020-01289-w