Abstract
The present work aimed to investigate the law of the conversion of glycerol with diethyl carbonate (DEC) over the highly efficient Ce–NiO catalyst prepared by co-precipitation method. The thermodynamic and kinetics parameters of this reaction for glycerol carbonate synthesis were studied. The reaction equilibrium constants were investigated experimentally in the temperature region of 338–358 K, and the standard molar properties (at 298 K) were obtained (△H0= 115.71 kJ mol−1, △G0= 13.523 kJ mol−1, △S0= 0.343 kJ mol−1). Effects of various experimental conditions including stirring speed, reaction temperature, catalyst amount, reactant mole ratio on the reaction kinetics were also studied. Different order kinetic equations were used to simulate law of the transesterification process. The result shows a three-order equation of glycerol (two orders) and DEC (one order) was more appropriate to interpret the law of glycerol transesterification with diethyl carbonate. The activation energy of this reaction was obtained with 87.90 kJ mol−1. Finally, the microstructure and physicochemical properties of the Ce–NiO samples were studied by XRD, SEM, TEM, and CO2-TPD.
Graphical abstract
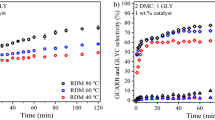
One-pot catalytic conversion of glycerol into glycerol carbonate over Ce–NiO catalyst.





Reproduced with permission (Wu et al. 2017). Copyright 2017, ELSEVIER Publications





Similar content being viewed by others
Abbreviations
- \(\alpha\) :
-
Reaction order of glycerol
- \(\beta\) :
-
Reaction order of diethyl carbonate
- \(c_{{{\text{A}}_{0} }}\) :
-
Initial concentration of glycerol (mol L−1)
- \(c_{{{\text{B}}_{ 0} }}\) :
-
Initial concentration of diethyl carbonate (mol L−1)
- \(c_{\text{A}}\) :
-
Concentration of glycerol after time t (mol L−1)
- \(c_{\text{B}}\) :
-
Concentration of diethyl carbonate after time t (mol L−1)
- \(c_{\text{C}}\) :
-
Concentration of glycerol carbonate after time t (mol L−1)
- \(c_{\text{D}}\) :
-
Concentration of ethanol after time t (mol L−1)
- \(\theta\) :
-
Mole ratio of \(c_{{{\text{A}}_{0} }}\) to \(c_{{{\text{B}}_{0} }}\)
- \(k\) :
-
Rate constant
- \(t\) :
-
Reaction time (h)
- K C :
-
Equilibrium constant
- x G :
-
Equilibrium conversion of glycerol (%)
- \(x\) :
-
Conversion of glycerol (%)
References
Alok KS, Fernando SD (2007) Reaction kinetics of soybean oil transesterification using heterogeneous metal oxide catalysts. Chem Eng Technol 30:1716–1720. https://doi.org/10.1002/ceat.200700274
Alvarez MG, Segarra AM, Contreras S, Sueiras JE, Medina F, Figueras F (2010) Enhanced use of renewable resources: transesterification of glycerol catalyzed by hydrotalcite-like compounds. Chem Eng J 161:340–345. https://doi.org/10.1016/j.cej.2009.12.036
Álvarez MG, Chimentão RJ, Figueras F, Medina F (2012a) Tunable basic and textural properties of hydrotalcite derived materials for transesterification of glycerol. Appl Clay Sci 58:16–24. https://doi.org/10.1016/j.clay.2012.02.004
Álvarez MG, Plíšková M, Segarra AM, Medina F, Figueras F (2012b) Synthesis of glycerol carbonates by transesterification of glycerol in a continuous system using supported hydrotalcites as catalysts. Appl Catal B 113–114:212–220. https://doi.org/10.1016/j.apcatb.2011.11.040
Aresta M, Dibenedetto A, Nocito F, Pastore C (2006) A study on the carboxylation of glycerol to glycerol carbonate with carbon dioxide: the role of the catalyst, solvent and reaction conditions. J Mol Catal A Chem 257:149–153. https://doi.org/10.1016/j.molcata.2006.05.021
Choi JS, Simanjuntaka FSH, Oh JY, Lee KI, Lee SD, Cheong M, Kim HS, Lee H (2013) Ionic-liquid-catalyzed decarboxylation of glycerol carbonate to glycidol. J Catal 297:248–255. https://doi.org/10.1016/j.jcat.2012.10.015
Climent MJ, Corma A, De Frutos P, Iborra S, Noy M, Velty A, Concepción P (2010) Chemicals from biomass: synthesis of glycerol carbonate by transesterification and carbonylation with urea with hydrotalcite catalysts. The role of acid–base pairs. J Catal 269:140–149. https://doi.org/10.1016/j.jcat.2009.11.001
Diaz-Alvarez AE, Francos J, Lastra-Barreira B, Crochet P, Cadierno V (2011) Glycerol and derived solvents: new sustainable reaction media for organic synthesis. Chem Commun 47:6208–6227. https://doi.org/10.1039/C1CC10620A
Dibenedetto A, Angelini A, Aresta M, Ethiraj J, Fragale C, Nocito F (2011) Converting wastes into added value products: from glycerol to glycerol carbonate, glycidol and epichlorohydrin using environmentally friendly synthetic routes. Tetrahedron 67:1308–1313. https://doi.org/10.1016/j.tet.2010.11.070
Dieuzeide ML, Amadeo N (2010) Thermodynamic analysis of glycerol steam reforming. Chem Eng Technol 33:89–96. https://doi.org/10.1002/ceat.200900260
Esteban J, Domínguez E, Ladero M, Garcia-Ochoa F (2015a) Kinetics of the production of glycerol carbonate by transesterification of glycerol with dimethyl and ethylene carbonate using potassium methoxide, a highly active catalyst. Fuel Process Technol 138:243–251. https://doi.org/10.1016/j.fuproc.2015.06.012
Esteban J, Fuente E, Blanco A, Ladero M, Garcia-Ochoa F (2015b) Phenomenological kinetic model of the synthesis of glycerol carbonate assisted by focused beam reflectance measurements. Chem Eng J 260:434–443. https://doi.org/10.1016/j.cej.2014.09.039
Esteban J, Ladero M, García-Ochoa F (2015c) Kinetic modelling of the solventless synthesis of solketal with a sulphonic ion exchange resin. Chem Eng J 269:194–202. https://doi.org/10.1016/j.cej.2015.01.107
Esteban J, Ladero M, Fuente E, Blanco Á, García-Ochoa F (2016) Experimental and modelling approach to the catalytic coproduction of glycerol carbonate and ethylene glycol as a means to valorise glycerol. J Taiwan Inst Chem Eng 63:89–100. https://doi.org/10.1016/j.jtice.2016.03.031
Fang W, Pirez C, Capron M, Paul S, Raja T, Dhepe PL, Dumeignil F, Jalowiecki-Duhamel L (2012) Ce–Ni mixed oxide as efficient catalyst for H2 production and nanofibrous carbon material from ethanol in the presence of water. RSC Adv 2:9626–9634. https://doi.org/10.1039/C2RA21701E
Faria RPV, Pereira CSM, Silva VMTM, Loureiro JM, Rodrigues AE (2013) Glycerol valorization as biofuel: thermodynamic and kinetic study of the acetalization of glycerol with acetaldehyde. Ind Eng Chem Res 52:1538–1547. https://doi.org/10.1021/ie302935w
Farias AFF, Moura KF, Souza JKD, Lima RO, Nascimento JDSS, Cutrim AA, Longo E, Araujo AS, Carvalho-Filho JR, Souza AG, Santos IMG (2015) Biodiesel obtained by ethylic transesterification using CuO, ZnO and CeO2 supported on bentonite. Fuel 160:357–365. https://doi.org/10.1016/j.fuel.2015.07.102
George J, Patel Y, Pillai SM, Munshi P (2009) Methanol assisted selective formation of 1,2-glycerol carbonate from glycerol and carbon dioxide using nBu2SnO as a catalyst. J Mol Catal A Chem 304:1–7. https://doi.org/10.1016/j.molcata.2009.01.010
Hakim L, Yaakob Z, Ismail M, Daud W, Sari R (2013) Hydrogen production by steam reforming of glycerol over Ni/Ce/Cu hydroxyapatite-supported catalysts. Chem Pap 67:703–712. https://doi.org/10.2478/s11696-013-0368-y
Hu J, Li J, Gu Y, Guan Z, Mo W, Ni Y, Li T, Li G (2010) Oxidative carbonylation of glycerol to glycerol carbonate catalyzed by PdCl2(phen)/KI. Appl Catal A 386:188–193. https://doi.org/10.1016/j.apcata.2010.07.059
Indran VP, Syuhada Zuhaimi NA, Deraman MA, Maniam GP, Yusoff MM, Yun Hin TY, Ab. Rahim MH (2014) An accelerated route of glycerol carbonate formation from glycerol using waste boiler ash as catalyst. RSC Adv 4:25257–25267. https://doi.org/10.1039/C4RA02910K
Kim DW, Park KA, Kim MJ, Kang DH, Yang JG, Park DW (2014) Synthesis of glycerol carbonate from urea and glycerol using polymer-supported metal containing ionic liquid catalysts. Appl Catal A 473:31–40. https://doi.org/10.1016/j.apcata.2013.12.032
Kondawar SE, Potdar AS, Rode CV (2015) Solvent-free carbonylation of glycerol with urea using metal loaded MCM-41 catalysts. RSC Adv 5:16452–16460. https://doi.org/10.1039/C4RA11590B
Lamers P, Hamelinck C, Junginger M, Faaij A (2011) International bioenergy trade—a review of past developments in the liquid biofuel market. Renew Sustain Energy Rev 15:2655–2676. https://doi.org/10.1016/j.rser.2011.01.022
Lee SD, Park MS, Kim DW, Kim I, Park DW (2014) Catalytic performance of ion-exchanged montmorillonite with quaternary ammonium salts for the glycerolysis of urea. Catal Today 232:127–133. https://doi.org/10.1016/j.cattod.2013.10.009
Lertlukkanasuk N, Phiyanalinmat S, Kiatkittipong W, Arpornwichanop A, Aiouache F, Assabumrungrat S (2013) Reactive distillation for synthesis of glycerol carbonate via glycerolysis of urea. Chem Eng Process 70:103–109. https://doi.org/10.1016/j.cep.2013.05.001
Li H, Gao D, Gao P, Wang F, Zhao N, Xiao F, Wei W, Sun Y (2013) The synthesis of glycerol carbonate from glycerol and CO2 over La2O2CO3–ZnO catalysts. Catal Sci Technol 3:2801–2809. https://doi.org/10.1039/C3CY00335C
Li H, Jiao X, Li L, Zhao N, Xiao F, Wei W, Sun Y, Zhang B (2015) Synthesis of glycerol carbonate by direct carbonylation of glycerol with CO2 over solid catalysts derived from Zn/Al/La and Zn/Al/La/M (M = Li, Mg and Zr) hydrotalcites. Catal Sci Technol 5:989–1005. https://doi.org/10.1039/C4CY01237B
Liu J, Daoutidis P, Yang B (2016) Process design and optimization for etherification of glycerol with isobutene. Chem Eng Sci 144:326–335. https://doi.org/10.1016/j.ces.2016.01.055
Molinero L, Ladero M, Tamayo JJ, Esteban J, García-Ochoa F (2013) Thermal esterification of cinnamic and p-methoxycinnamic acids with glycerol to cinnamate glycerides in solventless media: a kinetic model. Chem Eng J 225:710–719. https://doi.org/10.1016/j.cej.2013.04.016
Nakagawa Y, Tomishige K (2011) Catalyst development for the hydrogenolysis of biomass-derived chemicals to value-added ones. Catal Surv Asia 15:111–116. https://doi.org/10.1007/s10563-011-9114-z
Nanda MR, Yuan Z, Qin W, Ghaziaskar HS, Poirier MA, Xu CC (2014) Thermodynamic and kinetic studies of a catalytic process to convert glycerol into solketal as an oxygenated fuel additive. Fuel 117:470–477. https://doi.org/10.1016/j.fuel.2013.09.066
Nasreen S, Liu H, Qureshi LA, Sissou Z, Lukic I, Skala D (2016) Cerium–manganese oxide as catalyst for transesterification of soybean oil with subcritical methanol. Fuel Process Technol 148:76–84. https://doi.org/10.1016/j.fuproc.2016.02.035
Nemirowsky J (1885) Ueber die Einwirkung von Chlorkohlenoxyd auf Glycolchlorhydrin. Journal für Praktische Chemie 3:173–175. https://doi.org/10.1002/prac.18850310115
Ochoa-Gómez JR, Gómez-Jiménez-Aberasturi O, Maestro-Madurga B, Pesquera-Rodríguez A, Ramírez-López C, Lorenzo-Ibarreta L, Torrecilla-Soria J, Villarán-Velasco MC (2009) Synthesis of glycerol carbonate from glycerol and dimethyl carbonate by transesterification: catalyst screening and reaction optimization. Appl Catal A 366:315–324. https://doi.org/10.1016/j.apcata.2009.07.020
Padmanathan N, Selladurai S (2013) Electrochemical capacitance of porous NiO–CeO2 binary oxide synthesized via sol–gel technique for supercapacitor. Ionics 20:409–420. https://doi.org/10.1007/s11581-013-0989-8
Pandhare NN, Pudi SM, Mondal S, Pareta K, Kumar M, Biswas P (2017) Development of kinetic model for hydrogenolysis of glycerol over Cu/MgO catalyst in a slurry reactor. Ind Eng Chem Res 57:101–110. https://doi.org/10.1021/acs.iecr.7b03684
Patil P, Gude VG, Pinappu S, Deng S (2011) Transesterification kinetics of Camelina sativa oil on metal oxide catalysts under conventional and microwave heating conditions. Chem Eng J 168:1296–1300. https://doi.org/10.1016/j.cej.2011.02.030
Quispe CAG, Coronado CJR, Carvalho JA Jr (2013) Glycerol: production, consumption, prices, characterization and new trends in combustion. Renew Sustain Energy Rev 27:475–493. https://doi.org/10.1016/j.rser.2013.06.017
Sonnati MO, Amigoni S, Taffin de Givenchy EP, Darmanin T, Choulet O, Guittard F (2013) Glycerol carbonate as a versatile building block for tomorrow: synthesis, reactivity, properties and applications. Green Chem 15:283–306. https://doi.org/10.1039/C2GC36525A
Sun Y, Tong X, Wu Z, Liu J, Yan Y, Xue S (2014) A sustainable preparation of glycerol carbonate from glycerol and urea catalyzed by hydrotalcite-like solid catalysts. Energy Technol 2:263–268. https://doi.org/10.1002/ente.201300135
Vlad E, Bildea CS, Bozga G (2013) Robust optimal design of an glycerol etherification process. Chem Eng Technol 36:251–258. https://doi.org/10.1002/ceat.201200274
Wang L, Ma Y, Wang Y, Liu S, Deng Y (2011) Efficient synthesis of glycerol carbonate from glycerol and urea with lanthanum oxide as a solid base catalyst. Catal Commun 12:1458–1462. https://doi.org/10.1016/j.catcom.2011.05.027
Wong YC, Tan YP, Taufiq-Yap YH, Ramli I, Tee HS (2015) Biodiesel production via transesterification of palm oil by using CaO–CeO2 mixed oxide catalysts. Fuel 162:288–293. https://doi.org/10.1016/j.fuel.2015.09.012
Wu YF, Song XH, Cai FF, Xiao GM (2017) Synthesis of glycerol carbonate from glycerol and diethyl carbonate over Ce–NiO catalyst: the role of multiphase Ni. J Alloy Compd 720:360–368. https://doi.org/10.1016/j.jallcom.2017.05.292
Xu S, Yan X, Wang X (2006) Catalytic performances of NiO–CeO2 for the reforming of methane with CO2 and O2. Fuel 85:2243–2247. https://doi.org/10.1016/j.fuel.2006.03.022
Yadav GD, Chandan PA (2014) A green process for glycerol valorization to glycerol carbonate over heterogeneous hydrotalcite catalyst. Catal Today 237:47–53. https://doi.org/10.1016/j.cattod.2014.01.043
Zhang J, He D (2014) Lanthanum-based mixed oxides for the synthesis of glycerol carbonate from glycerol and urea. React Kinet Mech Catal 113:375–392. https://doi.org/10.1007/s11144-014-0739-6
Zou W, Ge C, Lu M, Wu S, Wang Y, Sun J, Pu Y, Tang C, Gao F, Dong L (2015) Engineering the NiO/CeO2 interface to enhance the catalytic performance for CO oxidation. RSC Adv 5:98335–98343. https://doi.org/10.1039/C5RA20466F
Acknowledgements
This work was financially supported by National Natural Science Foundation of china (nos. 21276050, 21676054, 21406034), Natural Science foundation of Jiangsu (no. BK20161415), Fundamental Research Funds for the central Universities (nos. 2242018K40041, 3207045414, 3207045101, 3207045426), Key Laboratory Open Fund of Jiangsu Province (JSBEM201409).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, J., Wu, Y., Song, X. et al. Thermodynamic and kinetic studies for synthesis of glycerol carbonate from glycerol and diethyl carbonate over Ce–NiO catalyst. Chem. Pap. 72, 2909–2919 (2018). https://doi.org/10.1007/s11696-018-0518-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-018-0518-3