Abstract
Purpose
Bariatric surgery is considered the main treatment option for patients with severe obesity. The objective of our study is to compare intra- and postoperative outcomes between the robotic and laparoscopic approaches within the sleeve gastrectomy (SG), duodenal switch (DS), and Roux-en-Y gastric bypass (RYGB).
Materials and Methods
The data from the MBSAQIP were collected for patients who underwent SG, DS, and RYGB between 2015 and 2021. The postoperative and procedural outcomes including 30-day morbidity and mortality as well as operation length were analyzed using regression models.
Results
Our analysis included 1,178,886 surgeries with SG comprising the majority (70%) followed by RYGB (28%) and DS (1%). Other than a higher adjusted risk of unplanned reoperation for robotic RYGB (relative risk (RR) 1.07) and a statistically significant higher rate of postoperative wound disruption in robotic SG for robotic surgery (RR 1.56), there were no statistically significant between-approach differences including infection, wound disruption, death, or reoperation for DS, RYGB, or SG. Our data showed no significant difference in anastomotic leak rate between laparoscopic and robotic approaches in either the DS (p = 0.521) or RYGB (p = 0.800) procedures. Across our study period, the median operation lengths decreased significantly per year for both the robotic SG and DS.
Conclusions
Robotic and laparoscopic bariatric surgical procedures have statistically similar 30-day patient outcomes. Robotic bariatric procedures do have significantly longer median operative times than laparoscopic procedures. The decision to use a robotic approach or laparoscopic approach should be made based upon surgeon experience and possibly cost.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obesity has become an epidemic in the United States with a prevalence rising to 41.9% in 2017 [1]. Bariatric surgery has been considered an important treatment option for patients with severe obesity (body mass index (BMI) > 35 with at least one related comorbidity or BMI > 40). The sleeve gastrectomy has been the most commonly performed bariatric surgery with the Roux-en-Y gastric bypass and duodenal switch also being regularly pursued surgical options [2]. The laparoscopic approach has been the standard of care and has a proven safety and efficacy record for all three procedures. However, due to challenges with the bariatric population such as limited intra-peritoneal space secondary to increased visceral fat content or hepatomegaly, surgeons continue to look towards alternative approaches, namely robotic surgery (RS).
Compared with the laparoscopic approach, the robotic platform offers several potential advantages including greater dexterity and precision with tissue manipulation [3]. Despite this theoretical advantage, controversy continues as to whether the robotic approach is similar in efficacy to the laparoscopic approach in practice. Numerous analyses exist comparing laparoscopic and robotic postoperative outcomes including studies which utilize large data registries such as the Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program (MBSAQIP) database. Findings suggest little difference in mortality, potentially meaningful higher rates on key morbidity outcomes (such as surgical site infection rate and leak rate as calculated from composite variables) for RS, and significantly longer operation times for the robotic approach [4, 5]. However, questions about the maximum efficiency of the robotic platform persist, as does the possibility of further advances within the RS approach that may come from increased experience and more widespread usage. As the rate of usage of robotic bariatric surgery increases, continued assessment of the efficacies of each approach is critical to ensure patients are provided with the best possible outcomes.
The objective of our study is to compare intra- and postoperative outcomes between the robotic and laparoscopic approaches within the sleeve gastrectomy, duodenal switch, and Roux-en-Y gastric bypass.
Materials and Methods
Data Source and Case Selection
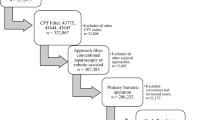
Data from the MBSAQIP between 2015 and 2021 were utilized to compare patients who underwent sleeve gastrectomy (SG), duodenal switch (DS), or Roux-en-Y gastric bypass (RYGB) and assess for differences in outcomes between the robotic-assisted and conventional laparoscopic-assisted approaches. The MBSAQIP data registry prospectively records more than 200 pre-, intra-, and postoperative variables using standardized definitions for all interventions performed at MBSAQIP-accredited centers in the United States and Canada; in 2020 and 2021, this represented 168,568 and 211,254 cases performed at 885 and 902 centers respectively [6]. MBSAQIP participant user data files include Health Insurance Portability and Accountability Act of 1996 (HIPPA) compliant patient-level data, and this retrospective cohort study was acknowledged as not human subjects research by the Institutional Review Board at Creighton University (record #2,003,469).
Cases meeting inclusion criteria were identified using Current Procedural Terminology (CPT) codes for SG (43,775), DS (43,845), and RYGB (43,644, 43,846,43,847) for the initial procedure. We then identified cases where the medical specialty of the physician performing the principal operative procedure was “Metabolic and Bariatric Surgeon,” or “General Surgeon” (combined these account for approximately 99% of cases), and identified whether the procedure was robotic or laparoscopic. These data were then merged with the 2015 to 2021 reoperation data in order to evaluate primary cohort outcomes such as reoperation.
Outcomes
Primary postoperative outcomes were mortality, reoperation (overall and unplanned), and wound disruption. The primary procedural outcome was operation length (minutes). Year-over-year trends were also evaluated for unplanned reoperation and operative length given a sufficient sample size. Secondary outcomes included postoperative superficial incisional surgical site infections (SSI), deep incisional SSI, organ space SSI, pneumonia, unplanned intubation, pulmonary embolism, ventilator use, progressive renal insufficiency, acute renal failure, urinary tract infection, stroke/cerebrovascular accident (CVA), cardiac arrest requiring cardiopulmonary resuscitation (CPR), myocardial infarction, venous thrombosis requiring therapy, sepsis, septic shock, unplanned admission to the intensive care unit (ICU), readmission, and days to death from operation. We also evaluated postoperative anastomotic leak rate, but this variable was only available beginning in 2020. All outcomes measured were within 30 days of surgery.
Covariate
For each surgery we abstracted age, biological sex, race, BMI (closest to bariatric surgery), smoking status, diabetes status, steroid use, chronic obstructive pulmonary disease (COPD), obstructive sleep apnea, previous foregut surgery, previous cardiac surgery, history of pulmonary embolism, hypertension, hyperlipidemia, history of deep vein thrombosis (DVT), American Society of Anesthesiologists (ASA) class, albumin levels, creatinine levels, renal insufficiency (pre-op), and dialysis.
Analysis
Differences in primary outcomes, as well as pre- and postoperative characteristics between laparoscopic and robotic approaches in SG, DS, and RYGB were compared. Continuous variables are presented as the median and interquartile range (IQR) and compared statistically using the Mann–Whitney U test. Categorical variables are presented as frequency and percentage and compared statistically using chi-square tests. Primary outcomes were compared using separate log-binomial regression models for each procedure to quantify the unadjusted and adjusted risk for each 30-day outcome between RS and laparoscopic approaches. For the adjusted models, the included covariates varied; potential covariates were excluded if there were inadequate expected frequencies (< 5) or missing data. Risk ratios are reported alongside 95% confidence intervals, with ratios greater than 1 indicating a greater risk of outcome for the robotic-assisted group. Finally, Poisson and log-binomial regression models were estimated for each procedure to evaluate differences in operative length and unplanned reoperation, respectively, to evaluate year-over-year differences between approaches. All analyses were conducted using statistical analysis software (SAS) v. 9.4 with two-tailed p < 0.05 used to indicate statistical significance.
Results
Postoperative Outcomes
Our analysis included 1,178,886 surgeries, with SG comprising the majority (70%), followed by RYGB (29%) and DS (1%). Univariate analyses of case demographics and preoperative comorbid conditions are presented in Table 1. Analysis of our primary outcomes is presented in Tables 2 and 3. Overall, the observed unplanned reoperation rates were slightly higher in RS for all surgery types. However, this difference was only statistically significant within RYGB (unadjusted 2.4% RS vs 2.2% laparoscopic, p = 0.003). The adjusted rates of unplanned reoperation for this comparison demonstrated a 7% higher risk of unplanned reoperation (relative risk (RR) 1.07, 95% confidence interval (CI) 1.01–1.14, p = 0.035, Table 3). Similarly, the observed difference in wound disruption rates between RS and laparoscopic surgery was generally low (≈ 0.1%). Within the SG, RS unadjusted rates of wound disruption were 0.1% compared with laparoscopic rates of < 0.1%; however, this difference was statistically significant, and results of the adjusted model showed a 56% higher risk of wound disruption for RS (RR 1.56, 95% CI 1.18–2.06; p = 0.002, Table 3). There were no additional statistically significant between-approach differences in infection, wound disruption, death, or reoperation for DS, RYGB, or SG (Table 3). Univariate analyses for secondary postoperative outcomes are presented in Table 4. Postoperative anastomotic leak, which was only available for 2020 and 2021 (73,981 total robotic and 278,661 total laparoscopic cases), showed no between-approach difference for DS (18/1648 (1.1%) vs 31/3,428 (0.9%), p = 0.521) or RYGB (69/22,866 (0.3%) vs 265/82,745 (0.3%), p = 0.800) but a significantly higher rate for RS in SG (94/49,467 (0.2%) vs 266/192,488 (0.1%), p = 0.020).
Median observed operation length (minutes) and unplanned reoperation rate (percent) between robotic and laparoscopic approaches by year for each procedure are presented in Fig. 1. For the DS, there was an observed decreasing trend for the laparoscopic approach (observed median time decreased from 127 min in 2015 to 120 min in 2021, ptrend < 0.001); a similar pattern was observed for the robotic approach (observed median time decreased from 220 min in 2015 to 163 min in 2021, ptrend < 0.001) though it was decreasing to a greater extent (pinteraction < 0.001). The same pattern was observed for the SG, with median laparoscopic time (68 min in 2015 to 57 min in 2021, ptrend < 0.001) decreasing, and median RS time (95 min in 2015 to 80 min in 2021, ptrend < 0.001) decreasing to a greater extent (pinteraction < 0.001).
Median observed operation length in minutes (top) and unplanned reoperation rate in percent (bottom) between robotic and laparoscopic approaches by year for each procedure. Operation length and unplanned reoperation over time. DS, duodenal switch; RYGB, Roux-en-Y gastric bypass; SG, sleeve gastrectomy
In modeling unplanned reoperation for the DS, there was an observed decreasing trend for laparoscopic surgery (3.36% in 2015 to 2.42% in 2021, ptrend < 0.003). A similar effect was observed for the robotic approach (5.56% in 2015 to 2.42% in 2021, ptrend < 0.007). This same pattern was observed for SG, with laparoscopic surgery (0.87% in 2015 to 0.53% in 2021, ptrend < 0.001) and RS (1.25% in 2015 to 0.68% in 2021, ptrend < 0.001) decreasing.
Discussion
Bariatric surgery has become an effective treatment option for obesity and laparoscopic surgery has been the gold standard approach for several years. Recently, robotic surgery has emerged as a potential alternative to laparoscopic surgery. Overall, when adjusted for comorbid conditions and preoperative variables, the robotic approach is statistically similar to the laparoscopic approach in regards to major 30-day postoperative outcomes. This is consistently observed across all three procedures (sleeve gastrectomy, gastric bypass, and duodenal switch). However, statistically significant differences were noted with respect to the length of the operation and reoperations, especially in RYGB. Our results are unique in comparing all three major bariatric surgeries in a single large database study. Incorporating the most recent years of available MBSAQIP data many of our study findings, such as operative length and readmissions, showed similar rates to previously reported MBSAQIP studies. However, unplanned return to the operating room has been a point of controversy [3,4,5, 7,8,9,10].
Our results showed a statistically significant higher rate of unplanned reoperations within 30 days in robotic RYGB as well as a higher rate of postoperative wound disruption in robotic SG. These postoperative outcomes are consistent with prior literature [11]. Robotic surgery has shown similar trends in foregut surgery especially for hiatal hernia repairs in recent readmission database studies [12, 13]. Our data suggest that preoperative comorbid conditions such as COPD, prior foregut surgery, preoperative anticoagulation, and preoperative renal insufficiency, as well as characteristics including age, might be contributing slightly to higher reoperation rates in RYGB and higher readmission rates across all the robotic surgeries.
Postoperative bleeding and anastomotic-related complications, especially leaks, are considered the most common reasons for unplanned reoperations and morbidity. Our current dataset, which has leaks available from 2020, showed no significant difference between the laparoscopic and robotic approaches in either the DS or RYGB procedures. This is in part related to the lower total number of robotic DS and RYGB cases over 2 years (n = 1648 for DS and n = 22,866 for RYGB). Our results contrast with the retrospective study by Buchs N.C. et al. which showed that their robotic-assisted surgery group had a lower incidence of anastomotic leak (1.5% vs. 3.2%) and a shorter hospital stay (1.6 vs. 2.2 days) compared to the laparoscopic group [12, 13]. Our results showed similar leak rates between the procedures across platforms. Interestingly, SG (2020–2021 total n = 241,955) has a statistically significant higher rate (0.2% vs 0.1%) of leaks observed with the robotic approach. It is worth noting that, to our knowledge, this study includes the largest sample comparing leaks across the most common bariatric surgeries between approaches and is one of the initial reports comparing the leak rate for laparoscopic and robotic approaches across all major bariatric procedures in a large population database. As experience with RS has increased, the complications, especially leaks, have continued to show similar rates and trends when compared to the laparoscopic group.
The robotic approach has been proposed to be advantageous in patients with a high BMI classification as well as in technically difficult procedures like pancreatic surgery. In bariatric surgery, the DS is considered to be a technically challenging surgery, and robotic technology was adopted by some centers to show moderate benefits [14]. Our data showed that patients undergoing the robotic-assisted DS demonstrated a significantly higher rate of preoperative venous thrombosis requiring therapy, higher rates of prior foregut surgeries, and significantly higher BMIs. The higher BMIs in the DS group compared to the SG and RYGB could be explained by preference among bariatric surgeons whereas the choice to use the robotic-assisted approach in patients with higher BMIs could be explained by the better maneuverability offered by the robotic approach in cases with decreased intra-peritoneal space [11]. Our data suggest that utilizing a robotic approach for the DS is at least not statistically different from the laparoscopic approach with similar rates of leaks and unplanned reoperations. This trend is seen despite a patient population with a slightly higher severity of obesity, an increased need for anticoagulant therapy, and an increased rate of prior foregut procedures when compared to the laparoscopic patient population.
Our study does coincide with the current literature suggesting that robotic bariatric procedures have a longer average operation length [4, 7,8,9, 14]. In our data, RS had significantly longer operative times for all three procedures. The largest median difference was seen in the robotic duodenal switch which on average took 75 more minutes than the laparoscopic approach. This difference was smaller within the RYGB (robotic approach took 34 min longer on average) and sleeve gastrectomy (robotic approach took 23 min longer on average). Robotic-assisted RYGB did not show any decrease in median operative lengths across our data period. However, the median operative lengths for robotic-assisted SG and DS continued to decrease significantly per year during our data collection. This finding could be indicative of a learning curve associated with the robotic-assisted bariatric surgical techniques. Over time, the robotic approach should continue to improve and bring operative lengths closer to those with the laparoscopic approach, which should also bring down the cost associated with robotic surgery. In a recent publication by Ayesha P. Ng et al. utilizing the National Inpatient Sample (NIS) database, the robotic approach is associated with higher overall cost compared to the laparoscopic approach in select abdominal procedures (elective gastrectomy, cholecystectomy, colectomy, ventral hernia repairs, hysterectomy, and abdominoperineal resection) [15]. Interestingly, this cost discrepancy widened throughout their data period, though they remark that the average age and comorbidity burden increased across the period in the robotic group as well which is consistent with our study results. The increasing cost is an interesting finding that seems to contrast with our findings of decreasing operative lengths per year. A possible explanation is that robotic technology has seen continuous improvement since its implementation and it could be that the newer platforms, while allowing for more efficient operations length-wise, have a higher initial investment that has led to increasing cost of use. The per-year decrease in median operative times for the robotic-assisted SG and DS suggests that the argument of cost should be made longitudinally rather than be compared to one snapshot analysis of procedure-related costs which has been the norm currently.
It is also important to highlight the technical differences between the robotic and laparoscopic anastomotic procedures. The anastomotic technique in RS more commonly involves suturing compared to a stapled anastomotic technique in the laparoscopic approach. Our data does not include details about intraoperative technique, but it has been debated as one of the important factors in long-term outcomes including weight loss, stricture, and anastomosis-related complications. Literature reports have shown significantly lower stricture rates with robotic surgery [10].
Our findings of higher unplanned reoperations in the robotic RYGB and a higher rate of wound disruption in the robotic SG beg the question of the clinical reasons behind these findings. Anastomotic leaks and bleeding are considered the most important reasons for reoperation; however, in our data, neither of these was significantly different between approaches, making it harder to elucidate the reasons behind these findings. Some patient factors such as COPD were higher in the robotic SG group, and this may have contributed to a higher rate of wound disruption, an association suggested in prior literature [16]. However, the absolute differences between our significant findings are small, and the most important clinical takeaway from this data is that there is no clear inferiority between the robotic and laparoscopic approaches in bariatric surgery with regard to patient safety.
Our retrospective analysis does have limitations, one being the lack of long-term follow-up data (limited to 30 days). Another limitation is the lack of data concerning the surgeon and the operating room team. Much of the current discourse regarding the efficacy of laparoscopic vs. robotic surgery concerns the lag between technological advancement and gaining procedural experience. As there is no way to discern how experienced a surgeon or institution is in the MBSAQIP data, we are unable to comment on how surgical experience may have influenced our results. To elucidate if these extended operative times when compared to the laparoscopic approach are inherent to the robotic platform or a short-term finding that will resolve as procedural experience with this new technology improves, a comparative study should be conducted evaluating postoperative bariatric surgical outcomes and operative times in the robotic-assisted approach adjusting for surgeon experience. This limitation also impacts the external validity of our study, as it is a fair assumption to say that a surgeon and operating team that primarily performs one type of bariatric procedure will be more efficient and efficacious than a surgeon who performs a wider variety of procedures with less individual frequency. Our analysis and discussion are comparing all three bariatric procedures to assess for any apparent inferiority; however, if a surgeon is significantly more comfortable and experienced with one procedure and platform over others, then it is unlikely that our generalized results will apply to their specific circumstance. There also was not enough volume of data for the DS to allow for an adjusted analysis of postoperative wound disruption or mortality. As the MBSAQIP database continues to add years of data, the outcomes of DS can be better assessed.
One more limitation that is found in our study as well as a large portion of bariatric surgery literature is the retrospective design. The highest quality study for the assessment of causal relationships would be a randomized controlled trial utilizing a large patient population. This is of course difficult in bariatric surgery as ultimately it is patient preference that dictates the type of procedure performed (SG, DS, RYGB) after a discussion with their surgeon. However, randomization for the usage of the robotic or laparoscopic platform, independent of what bariatric procedure is decided upon, is much more feasible. With a large sample size and a longer follow-up period, this would provide invaluable data in the discussion of laparoscopic vs. robotic surgery outcomes. This could also remove the limitation arising from the lack of knowledge of surgeon expertise; as this would be a prospective study with known participants, these metrics could be easily accounted for. It may even reveal how significant differences in outcomes are between surgeons of different experiences.
Conclusion
Robotic and laparoscopic bariatric surgical procedures have statistically similar 30-day patient outcomes. The robotic platform appears to be safe in the RYGB and DS despite a patient population with slightly increased rates of comorbidities and higher severities of obesity in the DS population. Robotic bariatric procedures do have significantly longer median operative times than laparoscopic procedures. Operative lengths have shown steady improvement with robotic surgery but remain high relative to the laparoscopic approach when utilizing the MBSAQIP database for comparison. Given the similarity in postoperative outcomes and continued improvement in operative times, the choice of approach should be dependent upon other factors such as surgeon experience and possibly overall cost.
Data Availability
MBSAQIP participant use data files are available only to employees (surgeons, researchers, bariatric program staff, etc.) of MBSAQIP-participant centers.
References
Centers for Disease Control and Prevention. (2022, May 17). Adult obesity facts. Retrieved August 28, 2022, from https://www.cdc.gov/obesity/data/adult.html
Mayo Foundation for Medical Education and Research. (2021, September 18). Bariatric surgery. Mayo Clinic. Retrieved August 28, 2022, from https://www.mayoclinic.org/tests-procedures/bariatric-surgery/about/pac-20394258#:~:text=Who%20it's%20for,pressure%20or%20severe%20sleep%20apnea.
Jung MK, Hagen ME, Buchs NC, et al. (2017). Robotic bariatric surgery: a general review of the current status.Int J Med Robotics Comp Assisted Surg, 13(4). https://doi.org/10.1002/rcs.1834
Fazl Alizadeh R, Li S, Inaba CS, et al. Robotic versus laparoscopic sleeve gastrectomy: a MBSAQIP analysis. Surg Endosc. 2019;33:917–22. https://doi.org/10.1007/s00464-018-6387-6.
Sebastian R, Howell MH, Chang KH, et al. Robot-assisted versus laparoscopic Roux-en-Y gastric bypass and sleeve gastrectomy: a propensity score-matched comparative analysis using the 2015–2016 MBSAQIP database. Surg Endosc. 2019;33:1600–12. https://doi.org/10.1007/s00464-018-6422-7.
ACS-MBSAQIP database (2015–2020) Metabolic and bariatric surgery accreditation and quality improvement program (MBSAQIP). Participant Use Data File (PUF) | ACS (facs.org). Accessed 17 March 2023.
Dudash M, Kuhn J, Dove J, et al. The longitudinal efficiency of robotic surgery: an MBSAQIP propensity matched 4-year comparison of robotic and laparoscopic bariatric surgery. Obes Surg. 2020;30:3706–13. https://doi.org/10.1007/s11695-020-04712-z.
Sharma G, Strong AT, Tu C, et al. Robotic platform for gastric bypass is associated with more resource utilization: an analysis of MBSAQIP dataset. Surg Obesity Related Diseas. 2018;14(3):304–10. https://doi.org/10.1016/j.soard.2017.11.018.
Scarritt T, Hsu CH, Maegawa FB, et al. Trends in utilization and perioperative outcomes in robotic-assisted bariatric surgery using the MBSAQIP database: a 4-year analysis. Obes Surg. 2021;31:854–61. https://doi.org/10.1007/s11695-020-05055-5.
Rogula T, Koprivanac M, Janik MR, et al. Does robotic Roux-en-Y gastric bypass provide outcome advantages over standard laparoscopic approaches? Obes Surg. 2018;28(9):2589–96. https://doi.org/10.1007/s11695-018-3228-6.PMID:29637410;PMCID:PMC6132787.
Anderson B, Gill RS, de Gara CJ, et al. (2013) Biliopancreatic diversion: the effectiveness of duodenal switch and its limitations. Gastroenterol Res Practice. 2013;974762:8. https://doi.org/10.1155/2013/974762.
Buchs NC, Pugin F, Bucher P, et al. Learning curve for robot-assisted Roux-en-Y gastric bypass. Surg Endosc. 2012;26(4):1116–21. https://doi.org/10.1007/s00464-011-2008-3.
Klock JA, Walters RW, Nandipati KC. Robotic hiatal hernia repair associated with higher morbidity and readmission rates compared to laparoscopic repair: 10-year analysis from the National Readmissions Database (NRD). J Gastrointest Surg. 2022;27(3):489–97. https://doi.org/10.1007/s11605-022-05548-x.
Clapp B, Liggett E, Jones R, et al. Comparison of robotic revisional weight loss surgery and laparoscopic revisional weight loss surgery using the MBSAQIP database. Surg Obesity Related Diseases. 2019;15(6):909–19. https://doi.org/10.1016/j.soard.2019.03.022.
Ng AP, Sanaiha Y, Bakhtiyar SS, et al. National analysis of cost disparities in robotic-assisted versus laparoscopic abdominal operations. Surgery. 2023;173(6):1340–5. https://doi.org/10.1016/j.surg.2023.02.016.
Shanmugam VK, Fernandez SJ, Evans KK, et al. (2015) Postoperative wound dehiscence: predictors and associations. Wound Repair Regen. 23(2):184–90. https://doi.org/10.1111/wrr.12268
Author information
Authors and Affiliations
Contributions
All authors met all four criteria for authorship as outlined by the International Committee of Medical Journal Editors.
Corresponding author
Ethics declarations
Ethics Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This project was acknowledged as not human subjects research by the Institutional Review Board at Creighton University (InfoEd record number, 2003469).
Consent to Participate
For this type of study, formal consent is not required. Informed consent does not apply.
Conflict of Interest
Clay L. Cashman, Swapnil V. Shah, Alexander G. Hall, and Ryan W. Walters — no conflict of interest. Kalyana C. Nandipati — proctor for Intuitive Surgical. The authors declare that they have no conflict of interest with the current project.
Additional information
Previous Presentations: Poster Presentations at the American College of Surgeons Nebraska Chapter on May 19, 2023, and the American Society for Metabolic and Bariatric Surgery on July 27, 2023.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key Points
• The robotic and laparoscopic approaches have similar outcomes in bariatric surgery.
• There is no significantly different rate of anastomotic leaks between approaches.
• Robotic bariatric surgery has longer operative times than the laparoscopic approach.
• The difference in operative times between approaches has been decreasing overall.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cashman, C.L., Shah, S.V., Hall, A.G. et al. Robotic-Assisted and Laparoscopic Bariatric Surgeries Still Have Clinically Comparable Outcomes. OBES SURG 34, 2954–2964 (2024). https://doi.org/10.1007/s11695-024-07368-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-024-07368-1