Abstract
Background
Despite the well-described optimal initial clinical response of sleeve gastrectomy (SG) in the treatment of obesity, some patients do not achieve optimal initial clinical response. Insulin-like growth factor-1 (IGF-1) has currently shown an association with post-bariatric surgery weight loss. This study aimed to assess the IGF-1 levels in female patients with obesity, the change after surgery, and their association with the metabolic profile and weight loss after surgery.
Patients and methods
This was a prospective study that was conducted on adult female patients who were recruited for SG. The patients underwent clinical and laboratory investigations that included the IGF-1 measurement. At the 1-year follow-up, the same clinical and laboratory measures were repeated.
Results
This study included 100 female patients. At the 1-year follow-up, there was a statistically significant reduction in body mass index (BMI) (p < 0.001), fasting HbA1C levels (p < 0.001), and triglycerides (p < 0.001), as well as a statistically significant increase in HDL (p < 0.001) and IGF-1 (p < 0.001). Multiple regression analysis revealed that, among the patients baseline characteristics, the significant predictors for the percentage of total weight loss (%TWL) were the patients’ BMI (p < 0.001) and IGF-1 levels (p < 0.001). The ROC curve showed that an IGF1 cutoff value of ≤ 23 ng/ml detected suboptimal initial clinical response, with a sensitivity of 95.35% and a specificity of 100%.
Conclusion
This study underscores the significant impact of SG on weight loss and metabolic improvements in female patients. Baseline IGF-1 levels emerged as a crucial predictor of optimal initial clinical response.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obesity is associated with several health disorders, resulting in a reduction in the quality of life and overall life expectancy [1, 2]. Achieving weight loss reduces the morbidity and mortality of patients with obesity [3]. Surgical treatment of obesity remains a reliable solution for patients with severe obesity to reduce their weight and improve the associated medical disorders [4].
This has resulted in a continuously growing number of bariatric surgeries performed annually all over the world [5]. Being a simple technique with promising safety and efficacy [6], sleeve gastrectomy (SG) is currently the most commonly performed bariatric surgery [5].
Despite the well-described optimal initial clinical response of SG in the treatment of obesity, some patients do not achieve optimal initial clinical response after surgery and require revisional bariatric surgery [7]. The outcome of SG has been described as being associated with various factors, including the patients’ age, sex, body mass index (BMI), and obesity complications [8,9,10,11,12,13,14].
Among the factors described, insulin-like growth factor-1 (IGF-1) is currently linked to post-bariatric surgery weight loss [13]. IGF-1 is one of the anabolic hormones that enhance energy expenditure [15, 16]. In addition, there is an intricate association between IGF-1 and growth hormone (GH), with the latter inducing the hepatic synthesis and secretion of the former [17], which subsequently reflect the GH secretion status [18] and mediate its lipolytic effects [19, 20]. Therefore, it could be proposed to be associated with weight loss following surgery.
However, data regarding the IGF-1 levels in patients with obesity and their change after surgery show conflicting results. Also, there remains scarce evidence regarding its potential effect on post-LSG weight loss.
The present study aimed to assess the IGF-1 levels in female patients with obesity, the change after surgery, and the association between these levels, the patient’s metabolic profile, and weight loss after surgery.
Patients and Methods
This was a prospective clinical study that was conducted on consecutive patients scheduled for LSG at our hospital during the period from December 2021 to December 2022 after obtaining Research Ethics Committee approval. The study followed the Helsinki Declaration.
After a multidisciplinary evaluation, patients’ eligibility for bariatric surgery was determined using criteria inspired by international societies concerned with obesity surgery [21,22,23]. Adult female patients who were recruited for LSG based on their choice after a thorough discussion with the surgeon, who presented the available choices, were included in the study. Patients with previous bariatric surgery and those who did not complete the follow-up visits were excluded from the study. The included patients provided informed written consent before being enrolled in the study.
The included patients underwent clinical assessment, including the anthropometric measures assessment and laboratory investigations that included the measurement of fasting serum glucose, glycosylated hemoglobin (HbA1c), lipid profile, and IGF-1, which was assessed with the Human Insulin-like Growth Factors 1, IGF-1ELISA Kit, SunLong Biotech Co., LTD, using the Elisa Plate Reader Statfax Chromate 4300 (U.S.A.). According to the manufacturer, normal IGF-1 levels range from 1.6 to 70 ng/mL.
The diagnostic criteria for diabetes are based on the measurement of blood glucose levels. According to the American Heart Association, a fasting level of 126 mg/dL or higher after an overnight fast of at least 8 h indicating diabetes mellitus [24]. The diagnostic criteria for hypertension are based on the measurement of blood pressure. According to the American Heart Association, a diagnosis of hypertension can be made in the following ways, a systolic blood pressure (SBP) of 130 mm Hg or higher or a diastolic blood pressure (DBP) of 80 mm Hg or higher on two or more readings taken on two or more occasions should diagnose hypertension [25]. The diagnostic criteria for dyslipidemia are based on the established guidelines, a diagnosis of dyslipidemia can be made when a total cholesterol level of 240 mg/dL or higher, a low-density lipoprotein (LDL) cholesterol level of 160 mg/dL or higher, and a high-density lipoprotein (HDL) cholesterol level of less than 40 mg/dL in men or less than 50 mg/dL in women [26].
Laparoscopic sleeve gastrectomy was performed as standardized [27]. In summary, the patients underwent routine preparation preoperatively, and the operation was conducted under general anesthesia. The required incisions were performed, and trocars were inserted. After the induction of pneumoperitoneum, the sleeved stomach pouch was created using a 36-Fr bougie. The stomach was resected starting from about 3–4 cm before the pyloric canal to the angle of His. Hemostasis was ensured throughout the surgery, and the leak testing was done. The patients received routine postoperative care and a schedule for postoperative visits.
At the 1-year follow-up, the same clinical and laboratory measures taken preoperatively were repeated. The total weight loss percentage (%TWL) was calculated [28]. A TWL percentage of less than 20% at the 1-year follow-up was considered suboptimal initial clinical response [29]. The resolution of the associated medical disorders was considered based on the American Society for Metabolic and Bariatric Surgery (ASMBS) recommendations. Remission of diabetes was considered if the HbA1c was less than 6 and fasting serum glucose was less than 100 mg/dL) in the absence of antidiabetic medications. Remission of hypertension was being normotensive (SBP < 120 mmHg and DBP < 80 mmHg). Remission of dyslipidemia was having LDL levels lower than < 100 mg/dL, triglycerides levels < 150 mg/dL, and HDL levels higher than 40 mg/dL [30].
Study Outcomes
The outcomes of the current work were the potential association of IGF-1 with the patients’ weight status and the predictors of weight loss after surgery. The secondary outcomes were the short-term outcomes of LSG.
Statistical Analysis
The patients’ data were analyzed using version 28 of the statistical software (SPSS, IBM Corp., Armonk, NY, USA). The patients’ data were expressed as a number and percentage if categorical, or a mean and standard deviation if numerical. An independent t test and a paired t test were used for the comparison of the numerical data, as appropriate. The McNemar’s test was used for the paired comparison of the categorical data. Pearson correlation analysis was used to test the correlation between numerical variables. Investigating the dynamic changes in IGF-1, the ∆ IGF (change from baseline) was calculated and its correlation with both %TWL and the change in Body Mass Index (∆ BMI) was explored. Multiple regression analysis was performed to assess the predictors for 1-year postoperative %TWL. The ROC curve was used to obtain the optimum IGF-1 cutoff value for the prediction of suboptimal initial clinical response. The level of significance was considered at a p-value of ≤ 0.05.
Results
One hundred female patients who completed the follow-up period were included in this study. Their mean age was 36.98 ± 10.05 years. The mean baseline weight was 136.12 ± 22.86 kg, the mean baseline BMI was 49.68 ± 8.13 kg/m2, and the mean baseline waist circumference (WC) was 111.64 ± 22.47 cm (Table 1). The patients’ obesity complications and baseline laboratory findings are presented in Table 1.
At baseline, the IGF-1 levels showed a significant negative correlation with the patients’ age (p < 0.001), WC (p = 0.023), fasting serum glucose levels (p < 0.001), and fasting serum HbA1C levels (p < 0.001) (Table 2).
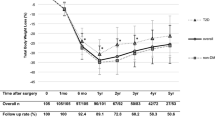
At the 1-year follow-up, there was a statistically significant reduction in weight (p < 0.001), BMI (p < 0.001), WC (p < 0.001), fasting glucose levels (p < 0.001), HbA1C levels (p < 0.001), and triglycerides (p < 0.001), and a statistically significant increase in HDL (p < 0.001) and IGF-1 (p < 0.001) (Table 1).
As for the obesity-associated medical disorders, complete resolution was shown in 47/56 patients with dyslipidemia (83.9%), 28/41 patients with hypertension (68.3%), and 15/32 patients with T2DM diabetes mellitus (46.9%). There was an improvement in the remaining patients with dyslipidemia (9/56; 16.1%), 6/41 patients with hypertension (14.6%), and 12/32 patients with T2DM (37.5%) (Table 1).
The 1-year weight loss was suboptimal (%TWL < 20%) in 14 patients (14%). Comparison of the patients’ baseline characteristics according to the 1-year weight loss showed that patients with suboptimal initial clinical response (%TWL < 20%) had significantly older age (p < 0.001), higher fasting serum glucose levels (p = 0.005), and lower IGF-1 levels (p < 0.001) (Table 3).
Comparison of the 1-year measures revealed that patients with suboptimal initial clinical response significantly higher weight and BMI (p < 0.001), fasting serum glucose levels (p = 0.003), HbA1C (p = 0.01), and triglycerides (p = 0.01), and significantly lower IGF-1 levels (p < 0.001) (Table 3).
The mean ∆ IGF-1 was 67.43 ± 25.88 ng/ml. there was statistically significant positive correlation between ∆ IGF-1 and %TWL (r = 0.716, p < 0.001) as well as ∆ IGF-1 and ∆ BMI (r = 0.211, p = 0.035).
Multiple regression analysis revealed that, among the patients baseline characteristics, the significant predictors for %TWL were the patients’ BMI (p < 0.001) and IGF-1 levels (p < 0.001). The effect of these parameters on %TWL was independent of the other patients’ parameters that were taken into account in the analysis (Table 4).
Testing the correlation of %TWL with the 1-year follow-up characteristics revealed that only the IGF-1 levels showed a statistically significant positive association (p < 0.001).
ROC curve analysis to determine the discriminant ability of the baseline IGF-1 in determining optimal 1-year weight loss showed that an IGF1 cutoff value of ≤ 23 ng/ml had excellent discriminant power to detect suboptimal initial clinical response, with an AUC of 0.975, a sensitivity of 95.35%, and a specificity of 100% (Fig. 1).
Discussion
Sleeve gastrectomy is a well-established bariatric procedure for patients with obesity who cannot achieve sustained weight loss with lifestyle modification or medical treatment [31,32,33]. The effect of SG is not only through the restriction of stomach volume, but other endocrinological pathways have been proposed to be a main interplay factor in the process of weight loss and the improvement of obesity complications [34]. The role of IGF-1 as an important metabolic regulator that reflects the levels of GH and partially mediates its growth effects has currently emerged [13, 35,36,37,38].
In this study, we investigated the SG effect on the metabolic profile of one hundred Egyptian adult female patients with obesity and the potential role of IGF-1. We selected female patients to provide a homogeneous study population, allowing us to minimize the impact of gender-related variations on IGF-1 levels. While IGF-1 is a crucial growth factor that plays diverse roles in both males and females, existing literature suggests that its circulating levels can vary between the sexes. Sex hormones, such as estrogen and testosterone, are known to influence IGF-1 production and regulation. Females tend to have different hormonal profiles than males, and these hormonal differences can contribute to variations in IGF-1 levels [39,40,41].
By focusing exclusively on female patients, we aimed to create a more homogenous study population, reducing the confounding effects of gender-related hormonal fluctuations on IGF-1 measurements. This approach enhances the internal validity of our findings and allows for a more focused investigation into the specific relationship between IGF-1 levels and weight loss outcomes in female patients undergoing sleeve gastrectomy.
The promising outcomes of LSG on weight loss and metabolic disorders are currently evidenced [31,32,33]. Our study emphasized this in terms of significant improvement in the lipid profile and glycemic control with high rates of resolution of associated medical disorders, besides the significant weight loss. This is supported by several previous studies that demonstrated a meaningful loss of weight and resolution of obesity-associated obesity complications after SG [31,32,33, 42, 43].
The SG-associated improvement of the metabolic parameters could be explained by the effect of reduced gastric volume on the amount of ingested food and the subsequent weight loss that was evidenced to affect the patients’ biochemical and metabolic parameters, including glucose levels and lipid profile [44]. However, other hormonal changes may also share the metabolic effect after LSG [36, 37, 45].
Among the possible explanations, IGF-1 might have an eminent role. Both insulin and IGF-1 have hypoglycemic and anabolic effects achieved by binding to the IGF-1 receptor and/or the insulin receptor [46], giving IGF-1 its own metabolic actions in insulin sensitivity, lipolysis, and proteolysis regulation as part of the IGF-1/insulin system [47]. Additionally, through IGF-1-mediated GH effects, the lipolysis process is stimulated by the free fatty acids released from fatty tissue, most prominently visceral fat. Moreover, hepatic triglyceride storage is maintained, and their uptake by skeletal muscles is stimulated [48]. It has been described that “fine tuning” of the IGF-1 signaling cascade is critical for proper adipogenesis [47].
IGF-1 has shown variable levels in patients with obesity, as previously described [13, 36, 49, 50]. In this study, despite being within the normal range, the preoperative IGF-1 levels were negatively correlated with the baseline WC, fasting glucose, and HbA1C levels. This elucidates the impact of abdominal obesity on IGF-1 levels and the subsequent disruption in glycemic control. The IGF-1 levels were also negatively correlated with the patients’ age. This correlation was previously described [51, 52] and explained by the reduction of GH levels in older individuals being nearly negligible in individuals aged above 60 years [52], with a subsequent decline in the hepatic production of IGF-1, which is regulated by GH.
In the present work, there was an evident elevation in the IGF-1 levels at the post-surgery follow-up. This is consistent with previous studies that reported a significant elevation in IGF-1 levels after bariatric surgery [13, 15, 35, 38, 53]. Interestingly, Mittempergher et al. [54] reported that IGF-1 levels did not exhibit significant elevation after bypass procedures, despite being significantly increased after SG. This was explained by the bypass procedure-associated deficiency in nutrients, including protein, which is needed for the improvement in IGF-1 levels after surgery [15]. However, Mittempergher et al. [54] found no significant association between baseline IGF-1 and postoperative weight loss.
Variable predictors for post-SG weight loss were described among studies [8,9,10,11,12,13,14]. Scarce conflicting evidence is available concerning the impact of IGF-1 levels on weight loss after SG [13, 54]. In this study, there was a significant association between ∆ IGF-1 and %TWL, pointing to the potential role of IGF-1 dynamics in influencing overall weight reduction. Moreover, after adjusting for the other confounders, BMI and IGF-1 were the baseline predictors for achieving optimal initial clinical response. This result implies the significant role of IGF-1 in the weight-loss process.
In line with our study, Ohira et al. [13] found that IGF-1 levels were a predictor for post-bariatric weight loss. Within the same context, Savastano et al. [55] found that the percentage of excess weight loss after surgery was significantly lower in patients with subnormal levels of IGF-1. The enhancing role of IGF-1 for weight loss after surgery could be explained by its anabolic role, which increases the mass of muscles, enhances the expenditure of energy, stimulates lipolysis, and regulates insulin sensitivity [15, 56]. It is worth noting that the current study showed a positive correlation of GLP-1 levels with the WC, reflecting the GH status. This finding could align with the described finding that GH causes a significant reduction in the amount of subcutaneous and visceral fat when it is used for the treatment of abdominal obesity [57].
Similar to our findings, researchers in previous studies have observed an obvious positive correlation between the baseline BMI values and the post-bariatric surgery weight loss [58,59,60].
While our results demonstrated a significant correlation between IGF-1 levels and various health parameters, including weight loss, it is crucial to consider the multifaceted nature of this relationship. It is imperative to recognize that correlation does not imply causation. The role of IGF-1 in metabolic regulation is complex and influenced by a myriad of factors, including nutritional status, hormonal balance, and physical activity levels [61]. Therefore, while our data suggest that IGF-1 levels may serve as a valuable biomarker in the context of weight loss, we cannot conclusively determine whether these levels are a direct causal factor, a mere indicator, or a consequence of weight loss and associated metabolic changes.
Furthermore, it is important to consider that patients with better weight loss outcomes often engage in more physical activity and have healthier dietary habits, which in turn could positively affect IGF-1 levels. This interplay highlights the importance of a holistic approach to understanding and interpreting the relationship between IGF-1 levels and weight loss. We acknowledge the need for further research to unravel the exact nature of this relationship. Future studies should aim to dissect the causal pathways and investigate how modifications in lifestyle factors could mediate the effects of IGF-1 on weight loss.
Investigating a cohort consisting exclusively of females, despite its potential limitation in generalizability, was a deliberate choice aimed at examining a more homogenous population. This approach allows for a focused analysis, minimizing the influence of gender-related variables that could confound the results. Another limitation of our study is the short-term follow-up period. Larger-scale studies with long-term follow-up are warranted to validate the clinical utility of baseline IGF-1 levels as a reliable marker for optimal initial clinical response. Furthermore, exploring the effectiveness of GLP-1 agonists in individuals with IGF-1 levels below a defined threshold presents an avenue for enhancing surgical outcomes.
Conclusion
This study underscores the significant impact of LSG on weight loss and metabolic improvements in female patients. Notably, baseline IGF-1 levels emerged as a crucial predictor for optimal initial clinical response, emphasizing their potential as a valuable marker in guiding clinical decisions and predicting surgical outcomes in this cohort. However, it is crucial to acknowledge that our results should be interpreted with caution due to the inherent limitations of our study. The association between IGF-1 levels and weight loss outcomes, while compelling, warrants further investigation to fully understand its nature and implications.
Data Availability
The datasets analyzed during the current study are available upon an editorial request.
References
Haslam DW, James WP. Obesity Lancet. 2005;366(9492):1197–209. https://doi.org/10.1016/S0140-6736(05)67483-1
GBD 2015 Obesity Collaborators, Afshin A, Forouzanfar MH, Reitsma MB, Sur P, Estep K, et al. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. 2017;377(1):13–27.
Gloy VL, Briel M, Bhatt DL, et al. Bariatric surgery versus non-surgical treatment for obesity: a systematic review and meta-analysis of randomised controlled trials. BMJ. 2013;347.
Welbourn R, Hollyman M, Kinsman R, et al. Bariatric surgery worldwide: baseline demographic description and one-year outcomes from the Fourth IFSO Global Registry Report 2018. Obes Surg. 2019;29(3):782–95.
Angrisani L, Santonicola A, Iovino P, et al. Bariatric surgery survey 2018: similarities and disparities among the 5 IFSO chapters. Obes Surg. 2021;31:1937–48.
Barqawi A, Abushamma FA, Akkawi M, et al. Global trends in research related to sleeve gastrectomy: a bibliometric and visualized study. World J Gastrointest Surg. 2021;13(11):1509–22.
Li S, Jiao S, Zhang S, et al. Revisional surgeries of laparoscopic sleeve gastrectomy. Diabetes Metab Syndr Obes. 2021;10(14):575–88.
Dixon JB, Dixon ME, O’Brien PE. Pre-operative predictors of weight loss at 1-year after Lap-Band surgery. Obes Surg. 2001;11:200–7.
Ma Y, Pagoto SL, Olendzki BC, et al. Predictors of weight status following laparoscopic gastric bypass. Obes Surg. 2006;16:1227–31.
Saboor Aftab SA, Halder L, Piya MK, et al. Predictors of weight loss at 1 year after laparoscopic adjustable gastric banding and the role of presurgical quality of life. Obes Surg. 2014;24:885–90.
Al-Khyatt W, Ryall R, Leeder P, et al. Predictors of inadequate weight loss after laparoscopic gastric bypass for morbid obesity. Obes Surg. 2017;27:1446–52.
Nickel F, de la Garza JR, Werthmann FS, et al. Predictors of risk and success of obesity surgery. Obes Facts. 2019;12:427–39.
Ohira M, Watanabe Y, Yamaguchi T, et al. Low serum insulin-like growth factor-1 level is a predictor of low total weight loss percentage after sleeve gastrectomy. Surg Obes Relat Dis. 2020;16:1978–87.
Sisik A, Basak F. Presurgical predictive factors of excess weight loss after laparoscopic sleeve gastrectomy. Obes Surg. 2020;30:2905–12.
Pellitero S, Granada ML, Martínez E, et al. IGF1 modifications after bariatric surgery in morbidly obese patients: potential implications of nutritional status according to specific surgical technique. Eur J Endocrinol. 2013;169(5):695–703.
Hussain MA, Schmitz O, Mengel A, et al. Insulin-like growth factor I stimulates lipid oxidation, reduces protein oxidation, and enhances insulin sensitivity in humans. J Clin Invest. 1993;92(5):2249–56.
Bergan-Roller HE, Sheridan MA. The growth hormone signaling system: insights into coordinating the anabolic and catabolic actions of growth hormone. Gen Comp Endocrinol. 2018;258:119–33.
Frystyk J. Free insulin-like growth factors − measurements and relationships to growth hormone secretion and glucose homeostasis. Growth Horm IGF Res. 2004;14(5):337–75.
Fain JN. Studies on the role of RNA and protein synthesis in the lipolytic action of growth hormone in isolated fat cells. Adv Enzyme Regul. 1967;5:39–51.
Sengupta K, Long KJ, Allen DO. Growth hormone stimulation of lipolysis and cyclic AMP levels in perifused fat cells. J Pharmacol Exp Ther. 1981;217(1):15.
Fried M, Hainer V, Basdevant A, et al. Interdisciplinary European guidelines on surgery of severe obesity. Obes Facts. 2008;1:52–9.
Fried M, Yumuk V, Oppert JM, European Association for the Study of Obesity; International Federation for the Surgery of Obesity - European Chapter, et al. Interdisciplinary European Guidelines on metabolic and bariatric surgery. Obes Facts. 2013;6(5):449–68.
Di Lorenzo N, Antoniou SA, Batterham RL, et al. Clinical practice guidelines of the European Association for Endoscopic Surgery (EAES) on bariatric surgery: update 2020 endorsed by IFSO-EC EASO and ESPCOP. Surg Endosc. 2020;34(6):2332–58.
American Diabetes Association. Standards of medical care in diabetes-2021 Abridged for Primary Care Providers. Clin Diabetes. 2021;39(1):14–43.
Ali A, Abu Zar M, Kamal A, Faquih AE, Bhan C, Iftikhar W, Malik MB, Ahmad MQ, Ali NS, Sami SA, Jitidhar F, Cheema AM, Zulfiqar A. American Heart Association High Blood Pressure Protocol 2017: A Literature Review. Cureus. 2018;10(8).
Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA /ACC /AACVPR /AAPA /ABC /ACPM /ADA /AGS /APhA /ASPC /NLA /PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139(25):e1082–143.
Ferrer-Márquez M, Belda-Lozano R, Ferrer-Ayza M. Technical controversies in laparoscopic sleeve gastrectomy. Obes Surg. 2012;22:182–7.
van de Laar AWJM, Acherman YIZ. Weight loss percentile charts of large representative series: a benchmark defining sufficient weight loss challenging current criteria for success of bariatric surgery. Obes Surg. 2014;24:727–34.
Seyssel K, Suter M, Pattou F, et al. A predictive model of weight loss after Roux-en-Y gastric bypass up to 5 years after surgery: a useful tool to select and manage candidates to bariatric surgery. Obes Surg. 2018;28(11):3393–9.
Brethauer SA, Kim J, el Chaar M, et al. Standardized outcomes reporting in metabolic and bariatric surgery. Surg Obes Relat Dis. 2015;11:489–506.
Bužga M, Holéczy P, Svagera Z, et al. Effects of sleeve gastrectomy on parameters of lipid and glucose metabolism in obese women - 6 months after operation. Wideochir Inne Tech Maloinwazyjne. 2013;8(1):22–8.
Wang L, Wang J, Jiang T. Effect of laparoscopic sleeve gastrectomy on type 2 diabetes mellitus in patients with body mass index less than 30 kg/m2. Obes Surg. 2019;29(3):835–42.
Wojciak PA, Pawłuszewicz P, Diemieszczyk I, et al. Laparoscopic sleeve gastrectomy: a study of efficiency in treatment of metabolic syndrome components, comorbidities and influence on certain biochemical markers. Wideochir Inne Tech Maloinwazyjne. 2020;15(1):136–47.
Liu FS, Wang S, Guo XS, et al. State of art on the mechanisms of laparoscopic sleeve gastrectomy in treating type 2 diabetes mellitus. World J Diabetes. 2023;14(6):632–55.
Galli G, Pinchera A, Piaggi P, et al. Serum insulin-like growth factor-1 concentrations are reduced in severely obese women and raise after weight loss induced by laparoscopic adjustable gastric banding. Obes Surg. 2012;22(8):1276–80.
Al-Regaiey K, Alshubrami S, Al-Beeshi I, et al. Effects of gastric sleeve surgery on the serum levels of GH, IGF-1 and IGF-binding protein 2 in healthy obese patients. BMC Gastroenterol. 2020;20(1):199.
Savastano S, Di Somma C, Barrea L, et al. The complex relationship between obesity and the somatropic axis: the long and winding road. Growth Horm IGF Res. 2014;24(6):221–6.
Pinto-Benito D, Paradela-Leal C, Ganchala D, et al. IGF-1 regulates astrocytic phagocytosis and inflammation through the p110α isoform of PI3K in a sex-specific manner. Glia. 2022;70(6):1153–69. https://doi.org/10.1002/glia.24163.
Harrela M, Koistinen H, Kaprio J, et al. Genetic and environmental components of interindividual variation in circulating levels of IGF-I, IGF-II, IGFBP-1, and IGFBP-3. J Clin Invest. 1996;98(11):2612–5.
Guerra-Cantera S, Frago LM, Díaz F, et al. Short-term diet induced changes in the central and circulating IGF systems are sex specific. Front Endocrinol (Lausanne). 2020;11(11):513.
Ohira M, Watanabe Y, Yamaguchi T, et al. The relationship between serum insulin-like growth factor-1 levels and body composition changes after sleeve gastrectomy. Obes Facts. 2021;14(6):641–9.
Sieber P, Gass M, Kern B, et al. Five-year results of laparoscopic sleeve gastrectomy. Surg Obes Relat Dis. 2014;10(2):243–9.
Albanopoulos K, Tsamis D, Natoudi M, et al. The impact of laparoscopic sleeve gastrectomy on weight loss and obesity-associated comorbidities: the results of 3 years of follow-up. Surg Endosc. 2016;30(2):699–705.
Askarpour M, Khani D, Sheikhi A, et al. Effect of bariatric surgery on serum inflammatory factors of obese patients: a systematic review and meta-analysis. Obes Surg. 2019;29(8):2631–47.
Bando H, Miura H, Kitahama S, et al. Preoperative serum cortisol level is predictive of weight loss after laparoscopic sleeve gastrectomy in men with severe obesity but not women. Obes Surg. 2023;33(3):851–9.
Yakar S, Adamo ML. Insulin-like growth factor 1 physiology: lessons from mouse models. Endocrinol Metab Clin N Am. 2012;41(2):231–47.
Kawai M, Rosen CJ. The IGF-I regulatory system and its impact on skeletal and energy homeostasis. J Cell Biochem. 2010;111:14–9.
Vijayakumar A, Novosyadlyy R, Wu Y, et al. Biological effects of growth hormone on carbohydrate and lipid metabolism. Growth Hormon IGF Res. 2010;20(1):1–7.
Maccario M, Grottoli S, Procopio M, et al. The GH/IGF-I axis in obesity: influence of neuro-endocrine and metabolic factors. Int J Obes Relat Metab Disord. 2000;24:S96–9.
Frystyk J. Free insulin-like growth factors—measurements and relationships to growth hormone secretion and glucose homeostasis. Growth Horm and IGF Res. 2004;14:337–75.
Bando H, Zhang C, Takada Y, et al. Impaired secretion of growth hormone-releasing hormone, growth hormone and IGF-I in elderly men. Acta Endocrinol. 1991;124:31–6.
Corpas E, Harman SM, Pineyro MA, et al. Continuous subcutaneous infusions of growth hormone (GH) releasing hormone 1–44 for 14 days increase GH and insulin-like growth factor-I levels in old men. J Clin Endocrinol Metabol. 1993;76:134–8.
Eden Engström B, Burman P, Holdstock C, et al. Effects of gastric bypass on the GH/IGF-I axis in severe obesity–and a comparison with GH deficiency. Eur J Endocrinol. 2006;154(2006):53–9.
Mittempergher F, Pata G, Crea N, et al. Preoperative prediction of growth hormone (GH)/insulin-like growth factor-1 (IGF-1) axis modification and postoperative changes in candidates for bariatric surgery. Obes Surg. 2013;23(5):594–601.
Savastano S, Angrisani L, Di Somma C, et al. Relationship between growth hormone/insulin-like growth factor-1 axis integrity and voluntary weight loss after gastric banding surgery for severe obesity. Obes Surg. 2010;20(2):211–20.
Hussain MA, Schmitz O, Mengel A, et al. Insulin-like growth factor I stimulates lipid oxidation, reduces protein oxidation, and enhances insulin sensitivity in humans. J Clin Invest. 1993;92(5):2249–56.
Hong JW, Park JK, Lim CY, et al. A weekly administered sustained-release growth hormone reduces visceral fat and waist circumference in abdominal obesity. Horm Metab Res. 2011;43(13):956–61.
Baltasar A, Perez N, Serra C, et al. Weight loss reporting: predicted body mass index after bariatric surgery. Obes Surg. 2011;21(3):367–72.
Goulart A, Leão P, Costa P, et al. Doctor, How Much Weight Will I Lose?-a New Individualized Predictive Model for Weight Loss. Obes Surg. 2016;26(6):1357–9.
Janik MR, Rogula TG, Mustafa RR, et al. Setting realistic expectations for weight loss after laparoscopic sleeve gastrectomy. Wideochir Inne Tech Maloinwazyjne. 2019;14(3):415–9.
Clemmons DR. Metabolic actions of insulin-like growth factor-I in normal physiology and diabetes. Endocrinol Metab Clin North Am. 2012;41(2):425–43, vii-viii.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
All authors equally contributed to this work.
Corresponding author
Ethics declarations
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Ethical Approval
This study has been approved by the appropriate institutional research ethics committee.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key points
• The study highlights the substantial impact of SG on weight loss and metabolic improvements among female patients with obesity.
• Baseline IGF-1 levels emerge as a crucial predictor for optimal initial clinical response, emphasizing the potential importance of this factor in assessing surgical outcomes.
• The study establishes an IGF-1 cutoff value of ≤ 23 ng/ml to detect suboptimal initial clinical response with high sensitivity (95.35%) and specificity (100%), providing a practical diagnostic tool.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Khattab, M.H., Said, S.M., Fayez, M.a. et al. The Association Between Preoperative Insulin-Like Growth Factor 1 Levels and the Total Body Weight Loss in Women Post Laparoscopic Sleeve Gastrectomy. OBES SURG 34, 874–881 (2024). https://doi.org/10.1007/s11695-024-07077-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-024-07077-9