Abstract
Purpose
Evidences about the gut microbiota role in weight loss after bariatric surgery (BS) are growing. The objective of this study was to observe the changes of gut microbiota after sleeve gastrectomy (SG) and SG plus truncal vagotomy (SG-TV) and identify specific microbes that may contribute to the improvement of obesity after surgeries.
Materials and Methods
Forty high-fat diet-induced obesity (DIO) mice were randomized to SG, SG-TV, or sham operation (SH) groups. Body weight (BW) and fast blood glucose (FBG) were measured before and 1, 2, 4, 8, and 12 weeks post-operatively. Fecal samples were collected before and at post-operative week 12 and profiled using 16S rRNA relative and absolute quantitative sequencing.
Results
After the surgery, the SG and SG-TV surgeries significantly reduce BW and FBG levels compared with SH, and the SG-TV achieved better effects than SG. A decreasing trend in alpha diversity of gut microbiota and significant changes in taxonomic composition were observed after surgeries. Then, we identified a set of microbes and pathways significantly different in abundance after BS. The genus Parabacteroides and one pathway (polyketide sugar unit biosynthesis) increased in SG-TV group specially, which was also negatively correlated with BW and FBG.
Conclusion
SG and SG-TV indeed achieve effects of weight loss, but TV could enhance the efficacy of SG. The identified different microbes and pathways, like Parabacteroides, polyketide sugar unit biosynthesis, may partly mediate the beneficial effects of BS, and thus possibly contribute to the development of novel bacteria-based therapeutic approaches.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The incidence of obesity is increasing at an unprecedented rate, and morbid obesity is currently a global health problem [1]. Bariatric surgery (BS) is currently the most effective treatment for obesity, and the application of sleeve gastrectomy (SG) exceeds 50% [2]. Besides, because of the role of vagus nerve in the regulation of appetite and obesity, it is increasingly recognized in the treatment of obesity [3].
Regardless of the method of BS, the gastrointestinal system will be affected, followed by changes of microbial community in the stomach and gut [4]. Several studies have evaluated the relations between gut microbiota changes after BS [5,6,7,8]. Animal and anthropological studies have reported the gut microbial changes after SG [9,10,11,12,13,14,15]. For example, Lu et al. found that the SG procedures lead to the increase of phylum Verrucomicrobia compared with sham group [10]. Sanchez-Alcoholado et al. found that the patients with severe obesity subjected in SG showed higher levels of Akkermansia, Eubacterium, Haemophilus, and Blautia [4]. However, controversial results were achieved from different literatures. Take phylum Proteobacteria for instance, some studies have shown a decrease in the abundance of Proteobacteria after SG, while some other studies concluded SG leads to an increase or no changes in Proteobacteria [5, 8, 13]. Given the controversial results of these researches, the present study will investigate the effects of SG on gut microbiota in DIO mice.
The vagus nerve is a central factor in the microbiome-gut-brain axis, microbes could communicate to the brain through the vagus nerve [16,17,18]. Evidences have showed that the vagus nerve could regulate eating behavior and BW [3, 19,20,21]. Actually, truncal vagotomy (TV) was promoted as a weight-loss procedure and resulted in good effects as early as 40 years ago [22, 23], although lost focus with the development of SG and Roux-en-Y gastric bypass (RYGB) subsequently. In recent years, with increasing attention of vagus nerve on weight regulating, several studies have reported that TV or SG-TV resulted in drastic reductions of BW [24, 25]. However, there is no study reported the effect of TV and TV-related BS on changing of gut microbiota. It remains to elucidate whether the vagus nerve and its related microbes could affect the effects of BS.
In this study, 40 high-fat diet-induced obesity (DIO) mice were used to perform different BSs, the body weight (BW) and fast blood glucose (FBG) were measured before and 1, 2, 4, 8, and 12 weeks post-operatively. The gut microbiota was also profiled before and after surgeries. We found SG and SG-TV indeed result in distinct changes in gut microbiota composition and metabolic pathways. And a specific genus Parabacteroides and one pathway (polyketide sugar unit biosynthesis), were identified to increase in the SG-TV groups, suggesting an important role in weight loss.
Materials and Methods
Animals
Ten-week-old male specific pathogen-free (SPF) mice (Jackson Laboratory, USA) were individually housed under a stable room temperature and relative humidity in a 12/12 h light/dark cycle. All the animals had free access to tap water and food unless otherwise stated. The mice were fed with a high-fat diet (Research Diets D12492, 60 kcal%) for 8 weeks to make them acquire diet-induced obesity (DIO). Subsequently, the DIO mice were divided to three groups randomly: SH (n = 11), SG (n = 19), and SG-TV (n = 10).
Surgical Interventions
Before the surgical operation, the mice were fasted 14 h with water available freely, and the BW and FBG levels were measured at baseline before surgery and on a weekly basis after surgery. Mice were anesthetized and then subjected to SH, SG, and SG-TV surgeries according to the group distribution. For SG, the procedure included a midline incision was made 1 cm below the xiphoid; the greater curvature of the stomach (approximately 80% of the gastric volume) was removed; the remnant stomach was closed using 7-0 silk sutures in a continuous manner. For SG-TV, in addition to the above operations, we made an incision in gastrohepatic ligament to expose the esophagus and the trunk of the vagus nerve, and then cutted off both the dorsal and ventral trunks above the point of bifurcation into the celiac and gastric or hepatic and accessory celiac branches, respectively. For sham operation (SH), the mice underwent laparotomy to expose the stomach, esophagus, and vagus trunk around the esophagus. No other procedures were carried out, but the exposure time and the incision were the same as SG and SG-TV groups.
Fecal Sampling, Processing, and Analysis
Fecal pellets were collected before and 12 weeks post-operatively. All the fecal samples were collected with sterile EP tubes and immediately stored at −80 °C prior to processing. The DNA of total bacteria in fecal samples were extracted with QIAamp Fast Stool DNA kit (Qiagen, Germany). The DNA samples were sent to Genesky Biological Technology Co., Ltd. (Shanghai, China) for 16S rRNA relative and absolute quantitative sequencing. The general description of the 16S rRNA absolute quantification sequencing was outlined in previous studies [26, 27]. Briefly, the V3-V4 region of bacteria 16S ribosomal RNA gene were chosen for amplification and sequenced on Illumina MiSeq. Nine different spike-in sequences with at least four different concentrations (103, 104, 105, and 106 of copies of internal standards) were added to the sample DNA pools. Spike-in sequences contained conserved regions identical to those of selected natural 16S rRNA genes and artificial variable regions different from nucleotide sequences in the public databases, which worked like internal standard and allowed the absolute quantification across samples.
Statistical Analysis
The data are expressed as the mean ± standard deviation (SD). BW and FBG levels were evaluated by using one-way analysis of variance (ANOVA), and statistical analyses were performed using IBM SPSS 19.0 version software. The Alpha diversity analysis and principal component analysis (PCA) were performed by R (version 4.0.5). The differential abundance of taxa between groups was identified through linear discriminant analysis (LDA) effect size (LEfSe) algorithm. Only taxon with LDA score > 2 or p < 0.05 (Kruskal–Wallis test) was considered significantly enriched.
Results
Different Surgeries Reduce BW and FBG
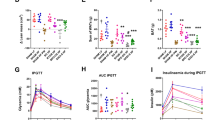
BW and FBG of mice were measured before and 1, 2, 4, 8, and 12 weeks post-operatively. We found the BW of mice steadily decreased, and the FBG level dropped dramatically in the first 2 weeks, then slowed down or rebound in 3 groups (Fig. 1). At week 12 post-operatively, the mean BW of mice in the SG-TV group was lowest (24.50 ± 2.52 g), followed by SG (33.62 ± 3.63 g), and SH group was the highest (43.29 ± 2.35 g). Moreover, a same trend was observed in the FBG level. Notably, the FBG level of mice in the SH group had a trend to recover to the pre-operative level.
Overall, the SG and SG-TV surgeries could achieve the effect of weight loss significantly, but the effect of SG-TV was better than that of SG, suggesting the synergistic effect of SG combined with TV in weight loss.
Different Surgeries Reduce the Diversity of Gut Microbiota
Diversity analysis showed a trend of decrease in bacterial diversity after surgeries. Simpson and Simpson indexes were significantly decreased after SG (p < 0.05) (Fig. 2A). PCA analysis indicated that the gut microbiota in the pre-surgery groups were distinctly dissimilar to that in the post-surgery groups (Fig. 2B), which indicated a great change of gut microbiota compositions caused by surgeries.
Different Surgeries Altered the Taxonomic Composition of Gut Microbiota
The composition of gut microbiota was analyzed from two perspectives: the relative abundances and absolute abundances of microbiota. The dominant phyla of gut microbiota were Bacteroidetes, Firmicutes, Proteobacteria, Actinobacteria, Candidatus Saccharibacteria and Verrucomicrobia (Fig. 3A). Absolute abundance analysis indicated that the absolute cell number obviously decreased in SH (0.85-fold) and SG (0.58-fold) groups, whereas increased in SG-TV group (1.13-fold), suggesting that TV may promote the proliferation of gut microbiota. But focused on specific phylum, we found that the total cell number of Proteobacteria and Verrucomicrobia increased apparently after SH. Similar results were observed at genus level (Fig. 3B), and the genera Anaerobacterium, Barnesiella and Parasutterella were found to decrease in SG-TV group after surgery. While for the relative abundance, some opposite results were observed. The relative abundance of phyla Bacteroidetes and Proteobacteria increased in SG group. Abundances of genus Alistipes increased in the SH group, while Anaerobacterium decreased in SG.
Considering that the absolute abundance could uncover the comprehensive dynamics of different bacterial communities, the subsequent analysis in this study was based on absolute abundance data [28].
The Microbiota Associated with BW and FBG
We further explored whether the change of gut microbiome have effects on weight loss and FBG decrease, and Pearson correlation analysis was conducted based on the data of 12 weeks post-operatively (Fig. 4). Weak correlations between BW or FBG and the abundance of gut microbiota were identified. Moreover, it is clear to see that the same correlation trend occurred in weight and FBG. At phylum level, Cyanobacteria/Chloroplast was the only phylum with significant correlation (weight r = 0.282, p = 0.011; FBG r = 0.233, p = 0.037) (Fig. 4A). At genus level, Eubacterium, Prevotella and Parabacteroides were the most negatively correlated with weight and FBG (Fig. 4B).
Enriched Microbes in Different BSs
In order to further investigate the impact of different surgeries on gut microbiota, LEfSe analysis was conducted to identify discriminative taxa. The taxas 32, 49, and 52 were identified in SH, SG, and SG-TV groups, respectively (Fig. 5A–C). Notable, there were several unique genera or phyla in SG and SG-TV groups (Fig. 5D, E). We found that genera Escherichia_Shigella and Trichococcus increased, and Oscillibacter decreased specifically in the SG group. Besides, phylum Proteobacteria and genera Parabacteroides, Fusimonas, Vampirovibrio and Bilophila increased, while genus Clostridium decreased specifically in the SG-TV group.
The microbiota profile differs between pre- and post-surgery samples in SH (A), SG (B), and SG-TV (C) group. Significance obtained by LDA score > 2. The Venn diagram compares and contrasts the number of taxa in phylum and genus levels that increased (D) and decreased (E) significantly after treatments with SH, SG, and SG-TV (all LDA score > 2). 1, pre-surgery; 2, post-surgery. Group 1: Bacteroidetes, Bacteroides; Group 2: Prevotella, Helicobacter, Anaerotruncus; Group 3: Firmicutes, Actinobacteria, Enterorhabdus
Among these different microbes, genera Escherichia_Shigella, Parabacteroides, and Vampirovibrio also showed negatively correlated with BW and FBG (Fig. 4), which suggested these microbes may be the potential contributors to stable weight loss and FBG reduced after bariatric surgery.
Results Compared with the Prior
To further confirm that these different microbes were indeed affected by surgery, we made a longitudinal comparison with related results based on sufficient literature research (Table 1). The results showed that changes of phyla Proteobacteria, Firmicutes and Bacteroidetes and genus Bacteroides varies in different studies, six genera including Vampirovibrio, Trichococcus, Helicobacter, Fusimonas, Escherichia_Shigella, Enterorhabdus, and Bilophila have not been confirmed in the prior studies, and change of genus Alistipes was inconsistent with previous studies. It is worth noting that changes of phylum Actinobacteria, genera Prevotella, Parabacteroides, Oscillibacter, Clostridium, and Anaerotruncus after surgeries were consistent with the present study, which indicated that these genera were indeed significantly affected by surgery. But considering that Parabacteroides was the only genus that is not present in the control group (SH) and showed negatively correlated with BW and FBG (Fig. 4), Parabacteroides may play important roles in post-operative weight loss.
Functional Analysis of Microbes after Different BS
Furthermore, we explored the effects of bariatric surgery on metabolism of gut microbiota. The KEGG pathways involved in each sample were predicted using the pircust2 software. A total of 76 pathways were identified to be significantly different before and after surgery process (Fig. 6). Heatmap showed that almost all KEGG pathways showed higher abundances after surgery in the SG-TV group, indicating an increasing activity of gut microbiota, and this result corroborated the above findings of the proliferation of gut microbiota after surgery. In addition, we found the abundances of polyketide sugar unit biosynthesis were higher in the SG-TV group, and Pearson correlation analysis also showed significant negatively correlated with BW (p = 0.025) and FBG (p = 0.016) (Fig. S1).
Discussion
There are increasing evidences for the role of gut microbes in post-operative weight loss [4]. In this study, we have explored the impacts of three BSs on weight loss and FBG level, as well as the short-term changes in gut microbiota composition and metabolic pathways after different BSs. It is worth noting that TV-related surgeries (SG-TV) were the first time to be studied. The beneficial effects of SG-TV were reinforced, and the changes in gut microbiota following SG-TV were studied firstly.
First, in addition to the FBG, F/B ratio was considered as an eventual biomarker of obesity [29, 30]. However, the validity of this potential marker is disputed. Some studies have reported that the composition of gut microbiota of obese subjects present a higher Firmicutes/Bacteroidetes ratio (F/B) [31,32,33]. Whereas, with the rapid accumulation of data, several studies did not find any differences of this parameter between obese and normal-weight individuals or even reported decreased F/B ratio in obese subjects [34, 35]. Furthermore, several meta-analyses have clarified that the heterogeneity of the results could be due to the insufficient number of subjects including in most of the studies [36, 37]. In our study, a trend was observed in decreasing F/B ratio after SG and SG-TV relative to pre-operation, while the opposite trend was found in mice which underwent SH. The inconsistency of results may be caused by different surgical methods. Murphy et al. examined gut microbiota changes after laparoscopic RYGB and SG in obese patients and found that RYGB resulted in increased F/B ratio, while SG resulted in the opposite result [12]. This may suggest that different surgical interventions are one of the main reasons for the heterogeneity of the results. Therefore, the ratio of F/B is not a robust marker of microbiome dysbiosis associated with obesity [38].
Second, little is known about the impact of TV-related surgeries on gut microbiota. In this study, results showed that the absolute cell number obviously increased after SG-TV, and the most discriminative taxa were identified in the SG-TV group. Notably, Parabacteroides was identified as the SG-TV specific genus, which deserves more attention. Previous studies have already suggested the potential role of Parabacteroides in obesity resistance [39, 40]. It was reported that Parabacteroides was negatively associated with multiple significant clinical indicators about bodyweight and serum lipids [39, 41]. Furthermore, Parabacteroides goldsteinii and Parabacteroides distasonis, belonging to the to the genus Parabacteroides have been considered as anti-obesity bacteria [42,43,44]. Previous studies have reported that P. goldsteinii and P. distasonis could play predominant role in the anti-obesity effects of related medicines, and are novel probiotic bacteria that may be used to treat obesity and associated metabolic disorders [43,44,45]. In addition, based on the predicted PICRUSt of gut microbiota, the present study revealed many pathways related to metabolisms. Among them, polyketide sugar unit biosynthesis shows higher abundance in the SG-TV group, and significantly negatively correlated with BW and FBG (Fig. 6; Fig. S1), which was also observed in previous studies [46, 47]. Fan et al. found that Parabacteroides and polyketide sugar unit biosynthesis were significantly increased at the same time, which was consistent with our results [48]. However, the potential correlation between them remains to be explored. In a word, the genus Parabacteroides may be a potential contributor to the beneficial effects of TV-related surgery, and the potential metabolic function need to be further verified.
We acknowledge several limitations of this study. Firstly, the sample size was limited but is comparable to other animal bariatric gut microbiota studies reported to date using 16S rRNA sequencing [10, 11]. Secondly, we use predicted rather than sequenced bacterial functions. Thus, metabolomics data are needed to explore these preliminary findings in further detail. Thirdly, the frequency of sampling collection is low; a long-term sampling was considered in our follow-up researches.
In conclusion, SG-TV could achieve a better effect of weight loss, indicating the synergistic effect of SG combined with TV. Besides, significant pre- to post-surgeries changes in the gut microbiome diversity, composition, and metabolic pathways were observed. And we found that the changes of the gut microbiome in a procedure-related manner. Moreover, the genus Parabacteroides, which is enriched specifically after SG-TV and negatively correlates with post-operative BW and FBG, may be the potential contributors to weight loss.
References
Collaboration NCDRF. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. Lancet. 2017;390(10113):2627–42.
Felsenreich DM, Bichler C, Langer FB, et al. Sleeve gastrectomy: surgical technique, outcomes, and complications. Surg Technol Int. 2020;36:63–9.
de Lartigue G. Role of the vagus nerve in the development and treatment of diet-induced obesity. J Physiol. 2016;594(20):5791–815.
Sanchez-Alcoholado L, Gutierrez-Repiso C, Gomez-Perez AM, et al. Gut microbiota adaptation after weight loss by Roux-en-Y gastric bypass or sleeve gastrectomy bariatric surgeries. Surg Obes Relat Dis Off J Am Soc Bariatric Surg. 2019;15(11):1888–95.
Davies NK, O'Sullivan JM, Plank LD, Murphy R. Altered gut microbiome after bariatric surgery and its association with metabolic benefits: a systematic review. Surg Obes Related Dis Off J Am Soc Bariat Surg. 2019;15(4):656–65.
Guo Y, Huang ZP, Liu CQ, et al. Modulation of the gut microbiome: a systematic review of the effect of bariatric surgery. Eur J Endocrinol. 2018;178(1):43–56.
Vangoitsenhoven R, Wilson R, Sharma G, et al. Metabolic effects of duodenojejunal bypass surgery in a rat model of type 1 diabetes. Surg Endosc. 2021;35(6):3104–14.
Shen N, Caixas A, Ahlers M, et al. Longitudinal changes of microbiome composition and microbial metabolomics after surgical weight loss in individuals with obesity. Surg Obes Relat Dis Off J Am Soc Bariat Surg. 2019;15(8):1367–73.
Karami R, Kermansaravi M, Pishgahroudsari M, et al. Changes in gut microbial flora after Roux-en-Y gastric bypass and sleeve gastrectomy and their effects on post-operative weight loss. Updat Surg. 2021;73(4):1493–9.
Lu C, Li Y, Li L, et al. Alterations of serum uric acid level and gut microbiota after Roux-en-Y gastric bypass and sleeve gastrectomy in a hyperuricemic rat model. Obes Surg. 2020;30(5):1799–807.
Shao Y, Ding R, Xu B, et al. Alterations of gut microbiota after Roux-en-Y Gastric Bypass and Sleeve Gastrectomy in Sprague-Dawley rats. Obes Surg. 2017;27(2):295–302.
Murphy R, Tsai P, Jullig M, et al. Differential changes in gut microbiota after gastric bypass and sleeve gastrectomy bariatric surgery vary according to diabetes remission. Obes Surg. 2017;27(4):917–25.
Campisciano G. Gut microbiota characterisation in obese patients before and after bariatric surgery Reply. Benef Microbes. 2018;9(6):998-.
Gutierrez-Repiso C, Molina-Vega M, Bernal-Lopez MR, et al. Different weight loss intervention approaches reveal a lack of a common pattern of gut microbiota changes. J Pers Med. 2021;11(2)
Yu D, Shu XO, Howard EF, et al. Fecal metagenomics and metabolomics reveal gut microbial changes after bariatric surgery. Surg Obes Relat Dis Off J Am Soc Bariat Surg. 2020;16(11):1772–82.
Miras AD, le Roux CW. Mechanisms underlying weight loss after bariatric surgery. Nat Rev Gastroenterol Hepatol. 2013;10(10):575–84.
Collins SM, Surette M, Bercik P. The interplay between the intestinal microbiota and the brain. Nat Rev Microbiol. 2012;10(11):735–42.
Bercik P, Collins SM, Verdu EF. Microbes and the gut-brain axis. Neurogastroenterol Motil. 2012;24(5):405–13.
Sarr MG, Billington CJ, Brancatisano R, et al. The EMPOWER study: randomized, prospective, double-blind, multicenter trial of vagal blockade to induce weight loss in morbid obesity. Obes Surg. 2012;22(11):1771–82.
Morton JM, Shah SN, Wolfe BM, et al. Effect of vagal nerve blockade on moderate obesity with an obesity-related comorbid condition: the recharge study. Obes Surg. 2016;26(5):983–9.
Klingbeil E, de La Serre CB. Microbiota modulation by eating patterns and diet composition: impact on food intake. Am J Phys Regul Integr Comp Phys. 2018;315(6):R1254–R60.
Kral JG. Vagotomy for treatment of severe obesity. Lancet. 1978;1(8059):307–8.
Kral JG. Effects of truncal vagotomy on body weight and hyperinsulinemia in morbid obesity. Am J Clin Nutr. 1980;33(2 Suppl):416–9.
Stearns AT, Balakrishnan A, Radmanesh A, et al. Relative contributions of afferent vagal fibers to resistance to diet-induced obesity. Dig Dis Sci. 2012;57(5):1281–90.
Liu T, Zhong MW, Liu Y, et al. Effects of sleeve gastrectomy plus trunk vagotomy compared with sleeve gastrectomy on glucose metabolism in diabetic rats. World J Gastroenterol. 2017;23(18):3269–78.
Tkacz A, Hortala M, Poole PS. Absolute quantitation of microbiota abundance in environmental samples. Microbiome. 2018;6(1):110.
Smets W, Leff JW, Bradford MA, et al. A method for simultaneous measurement of soil bacterial abundances and community composition via 16S rRNA gene sequencing. Soil Biol Biochem. 2016;96:145–51.
Jiang SQ, Yu YN, Gao RW, et al. High-throughput absolute quantification sequencing reveals the effect of different fertilizer applications on bacterial community in a tomato cultivated coastal saline soil. Sci Total Environ. 2019;687:601–9.
De Bandt JP, Waligora-Dupriet AJ, Butel MJ. Intestinal microbiota in inflammation and insulin resistance: relevance to humans. Curr Opin Clin Nutr Metab Care. 2011;14(4):334–40.
Zou Y, Ju X, Chen W, et al. Rice bran attenuated obesity via alleviating dyslipidemia, browning of white adipocytes and modulating gut microbiota in high-fat diet-induced obese mice. Food Funct. 2020;11(3):2406–17.
Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444(7122):1022–3.
de Wit N, Derrien M, Bosch-Vermeulen H, et al. Saturated fat stimulates obesity and hepatic steatosis and affects gut microbiota composition by an enhanced overflow of dietary fat to the distal intestine. Am J Physiol Gastrointest Liver Physiol. 2012;303(5):G589–99.
Hildebrandt MA, Hoffmann C, Sherrill-Mix SA, et al. High-fat diet determines the composition of the murine gut microbiome independently of obesity. Gastroenterology. 2009;137(5):1716-24 e1-2.
Tims S, Derom C, Jonkers DM, et al. Microbiota conservation and BMI signatures in adult monozygotic twins. ISME J. 2013;7(4):707–17.
Pajecki D, de Oliveira LC, Sabino EC, et al. Changes in the intestinal microbiota of superobese patients after bariatric surgery. Clinics. 2019;74:e1198.
Sze MA, Schloss PD. Looking for a signal in the noise: revisiting obesity and the microbiome. mBio. 2016;7(4)
Walters WA, Xu Z, Knight R. Meta-analyses of human gut microbes associated with obesity and IBD. FEBS Lett. 2014;588(22):4223–33.
Magne F, Gotteland M, Gauthier L, et al. The firmicutes/bacteroidetes ratio: a relevant marker of gut dysbiosis in obese patients? Nutrients. 2020;19:12(5).
Lv YR, Qin XX, Jia HJ, et al. The association between gut microbiota composition and BMI in Chinese male college students, as analysed by next-generation sequencing. Brit J Nutr. 2019;122(9):986–95.
Li S, You J, Wang Z, et al. Curcumin alleviates high-fat diet-induced hepatic steatosis and obesity in association with modulation of gut microbiota in mice. Food Res Int. 2021;143:110270.
Zeng Q, Li D, He Y, et al. Discrepant gut microbiota markers for the classification of obesity-related metabolic abnormalities. Sci Rep. 2019;9(1):13424.
Cai W, Xu J, Li G, et al. Ethanol extract of propolis prevents high-fat diet-induced insulin resistance and obesity in association with modulation of gut microbiota in mice. Food Res Int. 2020;130:108939.
Wu TR, Lin CS, Chang CJ, et al. Gut commensal Parabacteroides goldsteinii plays a predominant role in the anti-obesity effects of polysaccharides isolated from Hirsutella sinensis. Gut. 2019;68(2):248–62.
Xu Y, Wang N, Tan HY, et al. Panax notoginseng saponins modulate the gut microbiota to promote thermogenesis and beige adipocyte reconstruction via leptin-mediated AMPKalpha/STAT3 signaling in diet-induced obesity. Theranostics. 2020;10(24):11302–23.
Wang K, Liao M, Zhou N, et al. Parabacteroides distasonis alleviates obesity and metabolic dysfunctions via production of succinate and secondary bile acids. Cell Rep. 2019;26(1):222–35. e5
Li S, Zhuo M, Huang X, et al. Altered gut microbiota associated with symptom severity in schizophrenia. PeerJ. 2020;8:e9574.
Wang H, Hu C, Cheng C, et al. Unraveling the association of fecal microbiota and oxidative stress with stillbirth rate of sows. Theriogenology. 2019;136:131–7.
Fan Y, Ya EZ, Ji-Dong W, et al. Comparison of microbial diversity and composition in jejunum and colon of the alcohol-dependent rats. J Microbiol Biotechnol. 2018;28(11):1883–95.
Funding
This research was funded by the National Natural Science Foundation of China (Grant No.: 81570766) and Natural Science Foundation of Shanghai (Grant No.: 17ZR1438400).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethical Approval
This study was in accordance with Naval Medical University Ethics Committee. All applicable institutional and/or national guidelines for the care and use of animals were followed throughout the study.
Informed Consent
Informed consent does not apply.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key Points
1. SG combined with TV have a synergistic effect on weight loss;
2. SG and SG-TV reduced the diversity of gut microbiota;
3. Absolute abundance analysis indicated that TV may promote the proliferation of gut microbiota;
4. The genus Parabacteroides and one pathway (polyketide sugar unit biosynthesis) may partly mediate the beneficial effects of SG-TV, and thus possibly contribute to the development of novel bacteria-based therapeutic approaches.
Supplementary Information

Fig. S1
Correlation between postoperative weight or blood glucose and KEGG pathway. Only the KEGG pathway with significant differences were showed (p < 0.05) (PNG 82 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liang, D., Zhang, X., Liu, Z. et al. The Genus Parabacteroides Is a Potential Contributor to the Beneficial Effects of Truncal Vagotomy–Related Bariatric Surgery. OBES SURG 32, 1–11 (2022). https://doi.org/10.1007/s11695-022-06017-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-022-06017-9