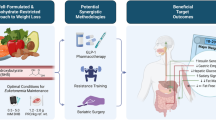
Abstract
In recent years, plenty of researches have reported in obese individuals with abnormal brain processes implicated in homeostatic regulation, reward, emotion, memory, attention, and executive function in eating behaviors. Thus, treating obesity cannot remain “brainless.” Behavioral and psychological interventions activate the food reward, attention, and motivation system, leading to minimal weight loss and high relapse rates. Pharmacotherapy is an effective weight loss method and regulate brain activity but with concerns about its brain function safety problems. Obesity surgery, the most effective therapy currently available for obesity, shows pronounced effects on brain activity, such as deactivation of reward and attention system, and activation of inhibition control toward food cues. In this review, we present an overview of alterations in the brain after the three common weight loss methods.




Similar content being viewed by others
References
Franz MJ, VanWormer JJ, Crain AL, et al. Weight-loss outcomes: a systematic review and meta-analysis of weight-loss clinical trials with a minimum 1-year follow-up. J Am Diet Assoc. 2007 Oct;107(10):1755–67.
Wolfenden L, Ezzati M, Larijani B, et al. The challenge for global health systems in preventing and managing obesity. Obes Rev. 2019;
Reilly JJ, Hughes AR, Gillespie J, et al. Physical activity interventions in early life aimed at reducing later risk of obesity and related non-communicable diseases: a rapid review of systematic reviews. Obes Rev. 2019;20(Suppl 1):61–73.
Murdaugh DL, Cox JE, Cook 3rd EW, et al. fMRI reactivity to high-calorie food pictures predicts short- and long-term outcome in a weight-loss program. NeuroImage. 2012;59(3):2709–21.
Fuentes Artiles R, Staub K, Aldakak L, et al. Mindful eating and common diet programs lower body weight similarly: systematic review and meta-analysis. Obes Rev. 2019;1
Krentz AJ, Fujioka K, Hompesch M. Evolution of pharmacological obesity treatments: focus on adverse side-effect profiles. Diabetes Obes Metab. 2016 Jun;18(6):558–70.
Sjostrom L. Review of the key results from the Swedish Obese Subjects (SOS) trial - a prospective controlled intervention study of bariatric surgery. J Intern Med. 2013;273(3):219–34.
Mikkelsen KH, Allin KH, Knop FK. Effect of antibiotics on gut microbiota, glucose metabolism and body weight regulation: a review of the literature. Diabetes Obes Metab. 2016;18(5):444–53. Epub 2016/01/29
Yao J, He Z, Chen Y, et al. Acupuncture and weight loss in Asians: a PRISMA-compliant systematic review and meta-analysis. Medicine. 2019;98(33):e16815.
Farr OM, Li CS, Mantzoros CS. Central nervous system regulation of eating: insights from human brain imaging. Metabolism. 2016;65(5):699–713.
Stice E, Burger K, Yokum S. Caloric deprivation increases responsivity of attention and reward brain regions to intake, anticipated intake, and images of palatable foods. NeuroImage. 2013;67:322–30.
Coulter AA, Rebello CJ, Greenway FL. Centrally acting agents for obesity: past, present, and future. Drugs. 2018;78(11):1113–32.
Baboumian S, Pantazatos SP, Kothari S, et al. Functional magnetic resonance imaging (fMRI) neural responses to visual and auditory food stimuli pre and post Roux-en-Y gastric bypass (RYGB) and sleeve gastrectomy (SG). Neuroscience. 2019;12
O'Brien PE, McPhail T, Chaston TB, et al. Systematic review of medium-term weight loss after bariatric operations. Obes Surg. 2006;16(8):1032–40.
Schwartz MW, Porte Jr D. Diabetes, obesity, and the brain. Science. 2005;307(5708):375–9.
Benoit SC, Air EL, Coolen LM, et al. The catabolic action of insulin in the brain is mediated by melanocortins. J Neurosci. 2002;22(20):9048–52.
Choudhury AI, Heffron H, Smith MA, et al. The role of insulin receptor substrate 2 in hypothalamic and beta cell function. J Clin Invest. 2005;115(4):940–50.
Cowley MA, Smart JL, Rubinstein M, et al. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature. 2001;411(6836):480–4.
Zigman JM, Bouret SG, Andrews ZB. Obesity impairs the action of the neuroendocrine ghrelin system. Trends Endocrinol Metab. 2016;27(1):54–63.
Maniscalco JW, Zheng H, Gordon PJ, et al. Negative energy balance blocks neural and behavioral responses to acute stress by “silencing” central glucagon-like peptide 1 signaling in rats. J Neurosci. 2015;35(30):10701–14.
van Bloemendaal L, Veltman DJ, ten Kulve JS, et al. Emotional eating is associated with increased brain responses to food-cues and reduced sensitivity to GLP-1 receptor activation. Obesity (Silver Spring). 2015;23(10):2075–82.
Skow MA, Bergmann NC, Knop FK. Diabetes and obesity treatment based on dual incretin receptor activation: ‘twincretins’. Diabetes Obes Metab. 2016;18(9):847–54.
Turer AT, Scherer PE. Adiponectin: mechanistic insights and clinical implications. Diabetologia. 2012;55(9):2319–26.
Bostrom PA, Fernandez-Real JM, Mantzoros C. Irisin in humans: recent advances and questions for future research. Metabolism. 2014;63(2):178–80.
Garcia-Garcia I, Horstmann A, Jurado MA, et al. Reward processing in obesity, substance addiction and non-substance addiction. Obes Rev. 2014;15(11):853–69.
DiLeone RJ, Taylor JR, Picciotto MR. The drive to eat: comparisons and distinctions between mechanisms of food reward and drug addiction. Nat Neurosci. 2012;15(10):1330–5.
Ziauddeen H, Alonso-Alonso M, Hill JO, et al. Obesity and the neurocognitive basis of food reward and the control of intake. Adv Nutr. 2015;6(4):474–86.
Volkow ND, Wang GJ, Tomasi D, et al. Obesity and addiction: neurobiological overlaps. Obes Rev. 2013;14(1):2–18.
Stice E, Spoor S, Bohon C, et al. Relation of reward from food intake and anticipated food intake to obesity: a functional magnetic resonance imaging study. J Abnorm Psychol. 2008;117(4):924–35.
Stice E, Yokum S, Bohon C, et al. Reward circuitry responsivity to food predicts future increases in body mass: moderating effects of DRD2 and DRD4. NeuroImage. 2010;50(4):1618–25.
Wang GJ, Volkow ND, Logan J, et al. Brain dopamine and obesity. Lancet. 2001;357(9253):354–7.
Volkow ND, Wang GJ, Telang F, et al. Low dopamine striatal D2 receptors are associated with prefrontal metabolism in obese subjects: possible contributing factors. NeuroImage. 2008;42(4):1537–43.
Gluck ME, Viswanath P, Stinson EJ. Obesity, appetite, and the prefrontal cortex. Curr Obes Rep. 2017;6(4):380–8.
Kullmann S, Heni M, Veit R, et al. Selective insulin resistance in homeostatic and cognitive control brain areas in overweight and obese adults. Diabetes Care. 2015;38(6):1044–50.
Reyes S, Peirano P, Peigneux P, et al. Inhibitory control in otherwise healthy overweight 10-year-old children. Int J Obes. 2015;39(8):1230–5.
Levitan RD, Rivera J, Silveira PP, et al. Gender differences in the association between stop-signal reaction times, body mass indices and/or spontaneous food intake in pre-school children: an early model of compromised inhibitory control and obesity. Int J Obes. 2015;39(4):614–9.
Nederkoorn C, Jansen E, Mulkens S, et al. Impulsivity predicts treatment outcome in obese children. Behav Res Ther. 2007;45(5):1071–5.
Doolan KJ, Breslin G, Hanna D, et al. Attentional bias to food-related visual cues: is there a role in obesity? Proc Nutr Soc. 2015;74(1):37–45.
Macht M. Characteristics of eating in anger, fear, sadness and joy. Appetite. 1999;33(1):129–39.
O'Doherty JP, Deichmann R, Critchley HD, et al. Neural responses during anticipation of a primary taste reward. Neuron. 2002;33(5):815–26.
Mehta S, Melhorn SJ, Smeraglio A, et al. Regional brain response to visual food cues is a marker of satiety that predicts food choice. Am J Clin Nutr. 2012;96(5):989–99.
Wang GJ, Yang J, Volkow ND, et al. Gastric stimulation in obese subjects activates the hippocampus and other regions involved in brain reward circuitry. Proc Natl Acad Sci U S A. 2006;103(42):15641–5.
Martin AA, Davidson TL. Human cognitive function and the obesogenic environment. Physiol Behav. 2014;136:185–93.
Parent MB, Darling JN, Henderson YO. Remembering to eat: hippocampal regulation of meal onset. Am J Physiol Regul Integr Comp Physiol. 2014;306(10):R701–13.
Loprinzi PD, Frith E. Obesity and episodic memory function. J Physiol Sci. 2018;68(4):321–31.
Thaler JP, Yi CX, Schur EA, et al. Obesity is associated with hypothalamic injury in rodents and humans. J Clin Invest. 2012;122(1):153–62.
Prada PO, Zecchin HG, Gasparetti AL, et al. Western diet modulates insulin signaling, c-Jun N-terminal kinase activity, and insulin receptor substrate-1ser307 phosphorylation in a tissue-specific fashion. Endocrinology. 2005;146(3):1576–87.
De Souza CT, Araujo EP, Bordin S, et al. Consumption of a fat-rich diet activates a proinflammatory response and induces insulin resistance in the hypothalamus. Endocrinology. 2005;146(10):4192–9.
Clegg DJ, Gotoh K, Kemp C, et al. Consumption of a high-fat diet induces central insulin resistance independent of adiposity. Physiol Behav. 2011;103(1):10–6.
Jais A, Bruning JC. Hypothalamic inflammation in obesity and metabolic disease. J Clin Invest. 2017;127(1):24–32.
Romanatto T, Cesquini M, Amaral ME, et al. TNF-alpha acts in the hypothalamus inhibiting food intake and increasing the respiratory quotient--effects on leptin and insulin signaling pathways. Peptides. 2007;28(5):1050–8.
Li J, Tang Y, Cai D. IKKbeta/NF-kappaB disrupts adult hypothalamic neural stem cells to mediate a neurodegenerative mechanism of dietary obesity and pre-diabetes. Nat Cell Biol. 2012;14(10):999–1012.
Chun SK, Jo YH. Loss of leptin receptors on hypothalamic POMC neurons alters synaptic inhibition. J Neurophysiol. 2010;104(5):2321–8.
Andre C, Dinel AL, Ferreira G, et al. Diet-induced obesity progressively alters cognition, anxiety-like behavior and lipopolysaccharide-induced depressive-like behavior: focus on brain indoleamine 2,3-dioxygenase activation. Brain Behav Immun. 2014;41:10–21.
Sobesky JL, Barrientos RM, De May HS, et al. High-fat diet consumption disrupts memory and primes elevations in hippocampal IL-1beta, an effect that can be prevented with dietary reversal or IL-1 receptor antagonism. Brain Behav Immun. 2014;42:22–32.
Paolicelli RC, Bolasco G, Pagani F, et al. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011 Sep 9;333(6048):1456–8.
Banks WA. Peptides and the blood-brain barrier. Peptides. 2015;72:16–9.
Banks WA. The blood-brain barrier as an endocrine tissue. Nat Rev Endocrinol. 2019;15(8):444–55.
Rhea EM, Salameh TS, Logsdon AF, et al. Blood-brain barriers in obesity. AAPS J. 2017;19(4):921–30.
Ouyang S, Hsuchou H, Kastin AJ, et al. Diet-induced obesity suppresses expression of many proteins at the blood-brain barrier. J Cereb Blood Flow Metab. 2014;34(1):43–51.
Karmi A, Iozzo P, Viljanen A, et al. Increased brain fatty acid uptake in metabolic syndrome. Diabetes. 2010;59(9):2171–7.
Andela S, Burrows TL, Baur LA, et al. Efficacy of very low-energy diet programs for weight loss: a systematic review with meta-analysis of intervention studies in children and adolescents with obesity. Obes Rev. 2019;20(6):871–82.
Astbury NM, Piernas C, Hartmann-Boyce J, et al. A systematic review and meta-analysis of the effectiveness of meal replacements for weight loss. Obes Rev. 2019;20(4):569–87.
Yen HY, Chiu HL. The effectiveness of wearable technologies as physical activity interventions in weight control: a systematic review and meta-analysis of randomized controlled trials. Obes Rev. 2019;20(10):1485–93.
Seo YG, Noh HM, Kim SY. Weight loss effects of circuit training interventions: a systematic review and meta-analysis. Obes Rev. 2019;19
Yang Y, Shields GS, Wu Q, et al. Cognitive training on eating behaviour and weight loss: a meta-analysis and systematic review. Obes Rev. 2019;28
Prehn K, Jumpertz von Schwartzenberg R, Mai K, et al. Caloric restriction in older adults-differential effects of weight loss and reduced weight on brain structure and function. Cereb Cortex. 2017;27(3):1765–78.
Michel S, Raab R, Drabsch T, et al. Do lifestyle interventions during pregnancy have the potential to reduce long-term postpartum weight retention? A systematic review and meta-analysis. Obes Rev. 2019;20(4):527–42.
Neumark-Sztainer D, Wall M, Guo J, et al. Obesity, disordered eating, and eating disorders in a longitudinal study of adolescents: how do dieters fare 5 years later? J Am Diet Assoc. 2006;106(4):559–68.
Stice E, Davis K, Miller NP, et al. Fasting increases risk for onset of binge eating and bulimic pathology: a 5-year prospective study. J Abnorm Psychol. 2008;117(4):941–6.
Doucet E, McInis K, Mahmoodianfard S. Compensation in response to energy deficits induced by exercise or diet. Obes Rev. 2018;19(Suppl 1):36–46.
Fuhrer D, Zysset S, Stumvoll M. Brain activity in hunger and satiety: an exploratory visually stimulated FMRI study. Obesity (Silver Spring). 2008;16(5):945–50.
Leidy HJ, Lepping RJ, Savage CR, et al. Neural responses to visual food stimuli after a normal vs. higher protein breakfast in breakfast-skipping teens: a pilot fMRI study. Obesity (Silver Spring). 2011;19(10):2019–25.
Goldstone AP, Prechtl de Hernandez CG, Beaver JD, et al. Fasting biases brain reward systems towards high-calorie foods. Eur J Neurosci. 2009;30(8):1625–35.
McDermott KD, Williams SE, Espeland MA, et al. Impact of intensive lifestyle intervention on neural food cue reactivity: action for health in diabetes brain ancillary study. Obesity (Silver Spring). 2019;27(7):1076–84.
Dong Z, Xu L, Liu H, et al. Comparative efficacy of five long-term weight loss drugs: quantitative information for medication guidelines. Obes Rev. 2017;18(12):1377–85.
Farr OM, Sofopoulos M, Tsoukas MA, et al. GLP-1 receptors exist in the parietal cortex, hypothalamus and medulla of human brains and the GLP-1 analogue liraglutide alters brain activity related to highly desirable food cues in individuals with diabetes: a crossover, randomised, placebo-controlled trial. Diabetologia. 2016;59(5):954–65.
van Bloemendaal L, Ten Kulve JS, la Fleur SE, et al. Effects of glucagon-like peptide 1 on appetite and body weight: focus on the CNS. J Endocrinol. 2014;221(1):T1–16.
Wadden TA, Hollander P, Klein S, et al. Weight maintenance and additional weight loss with liraglutide after low-calorie-diet-induced weight loss: the SCALE maintenance randomized study. Int J Obes. 2013;37(11):1443–51.
Davies MJ, Bergenstal R, Bode B, et al. Efficacy of Liraglutide for weight loss among patients with type 2 diabetes: the SCALE diabetes randomized clinical trial. JAMA. 2015;314(7):687–99.
Greenway FL, Fujioka K, Plodkowski RA, et al. Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2010;376(9741):595–605.
Apovian CM, Aronne L, Rubino D, et al. A randomized, phase 3 trial of naltrexone SR/bupropion SR on weight and obesity-related risk factors (COR-II). Obesity (Silver Spring). 2013;21(5):935–43.
Wadden TA, Foreyt JP, Foster GD, et al. Weight loss with naltrexone SR/bupropion SR combination therapy as an adjunct to behavior modification: the COR-BMOD trial. Obesity (Silver Spring). 2011;19(1):110–20.
Hollander P, Gupta AK, Plodkowski R, et al. Effects of naltrexone sustained-release/bupropion sustained-release combination therapy on body weight and glycemic parameters in overweight and obese patients with type 2 diabetes. Diabetes Care. 2013;36(12):4022–9.
Wang GJ, Tomasi D, Volkow ND, et al. Effect of combined naltrexone and bupropion therapy on the brain's reactivity to food cues. Int J Obes. 2014;38(5):682–8.
Roth BL, Willins DL, Kristiansen K, et al. 5-Hydroxytryptamine2-family receptors (5-hydroxytryptamine2A, 5-hydroxytryptamine2B, 5-hydroxytryptamine2C): where structure meets function. Pharmacol Ther. 1998;79(3):231–57.
Smith SR, Weissman NJ, Anderson CM, et al. Multicenter, placebo-controlled trial of lorcaserin for weight management. N Engl J Med. 2010;363(3):245–56.
Fidler MC, Sanchez M, Raether B, et al. A one-year randomized trial of lorcaserin for weight loss in obese and overweight adults: the BLOSSOM trial. J Clin Endocrinol Metab. 2011 Oct;96(10):3067–77.
O'Neil PM, Smith SR, Weissman NJ, et al. Randomized placebo-controlled clinical trial of lorcaserin for weight loss in type 2 diabetes mellitus: the BLOOM-DM study. Obesity (Silver Spring). 2012;20(7):1426–36.
Farr OM, Upadhyay J, Gavrieli A, et al. Lorcaserin administration decreases activation of brain centers in response to food cues and these emotion- and salience-related changes correlate with weight loss effects: a 4-week-long randomized, placebo-controlled, double-blind clinical trial. Diabetes. 2016;65(10):2943–53.
Colman E. Food and Drug Administration's obesity drug guidance document: a short history. Circulation. 2012;125(17):2156–64.
Sjostrom L, Peltonen M, Jacobson P, et al. Bariatric surgery and long-term cardiovascular events. JAMA. 2012;307(1):56–65.
Zhu C, Mei F, Gao J, et al. Changes in inflammatory markers correlated with increased testosterone after laparoscopic sleeve gastrectomy in obese Chinese men with acanthosis nigricans. J Dermatol. 2019;46(4):338–42.
Zhang Y, Zhu C, Wen X, et al. Laparoscopic sleeve gastrectomy improves body composition and alleviates insulin resistance in obesity related acanthosis nigricans. Lipids Health Dis. 2017;16(1):209.
Gao J, Zhang M, Zhu C, et al. The change in the percent of android and gynoid fat mass correlated with increased testosterone after laparoscopic sleeve gastrectomy in Chinese obese men: a 6-month follow-up. Obes Surg. 2018;28(7):1960–5.
Cardoso L, Rodrigues D, Gomes L, et al. Short- and long-term mortality after bariatric surgery: a systematic review and meta-analysis. Diabetes Obes Metab. 2017;19(9):1223–32.
Yeo C, Kaushal S, Lim B, et al. Impact of bariatric surgery on serum uric acid levels and the incidence of gout-A meta-analysis. Obes Rev. 2019;
Hansen TT, Jakobsen TA, Nielsen MS, et al. Hedonic changes in food choices following Roux-en-Y gastric bypass. Obes Surg. 2016;26(8):1946–55.
Gero D, Dib F, Ribeiro-Parenti L, et al. Desire for Core tastes decreases after sleeve Gastrectomy: a single-center longitudinal observational study with 6-month follow-up. Obes Surg. 2017;27(11):2919–26.
Alosco ML, Galioto R, Spitznagel MB, et al. Cognitive function after bariatric surgery: evidence for improvement 3 years after surgery. Am J Surg. 2014;207(6):870–6.
Behary P, Miras AD. Food preferences and underlying mechanisms after bariatric surgery. Proc Nutr Soc. 2015;74(4):419–25.
Ochner CN, Kwok Y, Conceicao E, et al. Selective reduction in neural responses to high calorie foods following gastric bypass surgery. Ann Surg. 2011;253(3):502–7.
Zoon HFA, de Bruijn SEM, Smeets PAM, et al. Altered neural responsivity to food cues in relation to food preferences, but not appetite-related hormone concentrations after RYGB-surgery. Behav Brain Res. 2018;353:194–202.
Scholtz S, Miras AD, Chhina N, et al. Obese patients after gastric bypass surgery have lower brain-hedonic responses to food than after gastric banding. Gut. 2014;63(6):891–902.
Frank S, Wilms B, Veit R, et al. Altered brain activity in severely obese women may recover after Roux-en Y gastric bypass surgery. Int J Obes. 2014;38(3):341–8.
Schauer PR, Bhatt DL, Kirwan JP, et al. Bariatric surgery versus intensive medical therapy for diabetes - 5-year outcomes. N Engl J Med. 2017;376(7):641–51.
Panagiotou OA, Markozannes G, Adam GP, et al. Comparative effectiveness and safety of bariatric procedures in Medicare-eligible patients: a systematic review. JAMA Surg. 2018;153(11):e183326.
Li G, Ji G, Hu Y, et al. Reduced plasma ghrelin concentrations are associated with decreased brain reactivity to food cues after laparoscopic sleeve gastrectomy. Psychoneuroendocrinology. 2019;100:229–36.
Bruce JM, Hancock L, Bruce A, et al. Changes in brain activation to food pictures after adjustable gastric banding. Surg Obes Relat Dis. 2012;8(5):602–8.
Mauro M, Papelbaum M, Brasil MAA, et al. Is weight regain after bariatric surgery associated with psychiatric comorbidity? A systematic review and meta-analysis. Obes Rev. 2019;20(10):1413–25.
Goldman RL, Canterberry M, Borckardt JJ, et al. Executive control circuitry differentiates degree of success in weight loss following gastric-bypass surgery. Obesity (Silver Spring). 2013;21(11):2189–96.
Li P, Shan H, Liang S, et al. Sleeve gastrectomy recovering disordered brain function in subjects with obesity: a longitudinal fMRI study. Obes Surg. 2018;28(8):2421–8.
Li G, Ji G, Hu Y, et al. Bariatric surgery in obese patients reduced resting connectivity of brain regions involved with self-referential processing. Hum Brain Mapp. 2018;39(12):4755–65.
le Roux CW, Aylwin SJ, Batterham RL, et al. Gut hormone profiles following bariatric surgery favor an anorectic state, facilitate weight loss, and improve metabolic parameters. Ann Surg. 2006;243(1):108–14.
Borg CM, le Roux CW, Ghatei MA, et al. Progressive rise in gut hormone levels after Roux-en-Y gastric bypass suggests gut adaptation and explains altered satiety. Br J Surg. 2006;93(2):210–5.
Abdeen G, le Roux CW. Mechanism underlying the weight loss and complications of Roux-en-Y gastric bypass. Rev Obes Surg. 2016;26(2):410–21.
van de Sande-Lee S, Pereira FR, Cintra DE, et al. Partial reversibility of hypothalamic dysfunction and changes in brain activity after body mass reduction in obese subjects. Diabetes. 2011;60(6):1699–704.
Gazdzinski SP, Gazdzinska AP, Orzel J, et al. Intragastric balloon therapy leads to normalization of brain magnetic resonance spectroscopic markers of diabetes in morbidly obese patients. NMR Biomed. 2018;31(9):e3957.
Dunn JP, Cowan RL, Volkow ND, et al. Decreased dopamine type 2 receptor availability after bariatric surgery: preliminary findings. Brain Res. 2010;1350:123–30.
Bruce AS, Bruce JM, Ness AR, et al. A comparison of functional brain changes associated with surgical versus behavioral weight loss. Obesity (Silver Spring). 2014;22(2):337–43.
Acknowledgments
We would like to thank Dr. Aaron M Gusdon (University of Texas Health Science Center at Houston) for discussion and critical reading of the manuscript.
Funding
The work was supported by the National Key R&D Program of China (No. 2018YFC1314100), National Natural Science Foundation of China (81970677), and National Natural Science Foundation of China for Youth (81500687).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval Statement
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent Statement
Does not apply.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lin, Z., Qu, S. Legend of Weight Loss: a Crosstalk Between the Bariatric Surgery and the Brain. OBES SURG 30, 1988–2002 (2020). https://doi.org/10.1007/s11695-020-04474-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-020-04474-8