Abstract
Background
Considering conflicting results on the consequences of all types of obesity surgery, we were to summarize them via a systematic review.
Methods
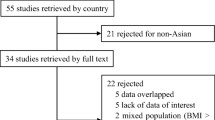
Electronic literature search was done via scientific search engines. After the removal of duplicates and selection of articles of interest, 771 studies were included.
Results
Insulin resistance indicators were significantly improved after bariatric surgery. Leptin was also significantly decreased while adiponectin was significantly increased. Although the level of metabolic hormones changed after bariatric surgery, they were not statistically significant. Inflammation indicators were significantly decreased. Significant reduction was also detected in PAI-1 and sICAM-1.
Conclusions
Bariatric surgery is beneficial in morbidly obese patients. Although treating obesity in a surgical way may cause some complications, the weight loss is generally safe and effective.





























Similar content being viewed by others
References
Yusuf S, Hawken S, Ôunpuu S, et al. Obesity and the risk of myocardial infarction in 27 000 participants from 52 countries: a case-control study. Lancet. 2005;366(9497):1640–9.
Wilson PW, D'agostino RB, Sullivan L, et al. Overweight and obesity as determinants of cardiovascular risk: the Framingham experience. Arch Intern Med. 2002;162(16):1867–72.
Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105(9):1135–43.
Ross R. Atherosclerosis-an inflammatory diseaseN Engl J Med 1999; 340: 2115-26. CrossRef Google Scholar 1999.
Blankenberg S, Barbaux S, Tiret L. Adhesion molecules and atherosclerosis. Atherosclerosis. 2003;170(2):191–203.
Lewczuk P, Dzienis-Str S, Kowalska I, et al. Elevated soluble intercellular adhesion molecule-1 levels in obesity: relationship to insulin resistance and tumor necrosis factor-[alpha] system activity. Metabolism. 2002;51(1):75–8.
Steffen B, Steffen L, Tracy R, et al. Obesity modifies the association between plasma phospholipid polyunsaturated fatty acids and markers of inflammation: the Multi-Ethnic Study of Atherosclerosis. Int J Obes. 2012;36(6):797–804.
Vaughan D. PAI-1 and atherothrombosis. J Thromb Haemost. 2005;3(8):1879–83.
Ito H, Ohshima A, Inoue M, et al. Weight reduction decreases soluble cellular adhesion molecules in obese women. Clin Exp Pharmacol Physiol. 2002;29(5–6):399–404.
Adami GF, Scopinaro N, Cordera R. Adipokine pattern after bariatric surgery: beyond the weight loss. Obes Surg. 2016;26(11):2793–801.
Finelli C, Padula MC, Martelli G, et al. Could the improvement of obesity-related co-morbidities depend on modified gut hormones secretion. World J Gastroenterol. 2014;20(44):16649–64.
Barja-Fernández S, Folgueira C, Castelao C, Leis R, Casanueva FF, Seoane LM. Peripheral signals mediate the beneficial effects of gastric surgery in obesity. Gastroenterol Res Pract 2015;2015.
Hydock CM. A brief overview of bariatric surgical procedures currently being used to treat the obese patient. Critical Care Nursing Quarterly. 2005;28(3):217–26.
Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1–34.
Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–5.
Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ: British Medical Journal. 2003;327(7414):557.
Sterne JA, Egger M, Smith GD. Systematic reviews in health care: investigating and dealing with publication and other biases in meta-analysis. BMJ (Clinical research ed). 2001;323(7304):101–5.
Vairavamurthy J, Cheskin LJ, Kraitchman DL, et al. Current and cutting-edge interventions for the treatment of obese patients. Eur J Radiol. 2017;93:134–42.
Chouillard E, Younan A, Alkandari M, et al. Roux-en-Y fistulo-jejunostomy as a salvage procedure in patients with post-sleeve gastrectomy fistula: mid-term results. Surg Endosc. 2016;30(10):4200–4.
Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292(14):1724–37.
Peterli R, Wölnerhanssen B, Peters T, et al. Improvement in glucose metabolism after bariatric surgery: comparison of laparoscopic Roux-en-Y gastric bypass and laparoscopic sleeve gastrectomy: a prospective randomized trial. Ann Surg. 2009;250(2):234–41.
de Moura EG, Martins BC, Lopes GS, et al. Metabolic improvements in obese type 2 diabetes subjects implanted for 1 year with an endoscopically deployed duodenal-jejunal bypass liner. Diabetes Technol Ther. 2012;14(2):183–9.
Wickremesekera K, Miller G, Naotunne TD, et al. Loss of insulin resistance after Roux-en-Y gastric bypass surgery: a time course study. Obes Surg. 2005;15(4):474–81.
Ballantyne G, Farkas D, Laker S, et al. Short-term changes in insulin resistance following weight loss surgery for morbid obesity: laparoscopic adjustable gastric banding versus laparoscopic Roux-en-Y gastric bypass. Obes Surg. 2006;16(9):1189–97.
Deitel M, Crosby RD, Gagner M. The first international consensus summit for sleeve gastrectomy (SG), New York City, October 25–27, 2007. Obes Surg. 2008;18(5):487–96.
Cummings DE, Overduin J, Shannon MH, et al. Hormonal mechanisms of weight loss and diabetes resolution after bariatric surgery. Surg Obes Relat Dis. 2005;1(3):358–68.
Camastra S, Gastaldelli A, Mari A, et al. Early and longer term effects of gastric bypass surgery on tissue-specific insulin sensitivity and beta cell function in morbidly obese patients with and without type 2 diabetes. Diabetologia. 2011;54(8):2093–102.
Rubino F, Schauer PR, Kaplan LM, et al. Metabolic surgery to treat type 2 diabetes: clinical outcomes and mechanisms of action. Annu Rev Med. 2010;61:393–411.
Dogan K, Betzel B, Homan J, et al. Long-term effects of laparoscopic Roux-en-Y gastric bypass on diabetes mellitus, hypertension and dyslipidaemia in morbidly obese patients. Obes Surg. 2014;24(11):1835–42.
Heptulla R, Smitten A, Teague B, et al. Temporal patterns of circulating leptin levels in lean and obese adolescents: relationships to insulin, growth hormone, and free fatty acids rhythmicity. The Journal of Clinical Endocrinology & Metabolism. 2001;86(1):90–6.
Considine RV, Sinha MK, Heiman ML, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334(5):292–5.
Gumbau V, Bruna M, Canelles E, et al. A prospective study on inflammatory parameters in obese patients after sleeve gastrectomy. Obes Surg. 2014;24(6):903–8.
Siejka A, Jankiewicz-Wika J, Kołomecki K, et al. Long-term impact of vertical banded gastroplasty (VBG) on plasma concentration of leptin, soluble leptin receptor, ghrelin, omentin-1, obestatin, and retinol binding protein 4 (RBP4) in patients with severe obesity. Cytokine. 2013;64(2):490–3.
Edwards C, Hindle AK, Fu S, et al. Downregulation of leptin and resistin expression in blood following bariatric surgery. Surg Endosc. 2011;25(6):1962–8.
van Dielen FM, van ‘tVeer C, Buurman WA, Greve JWM. Leptin and soluble leptin receptor levels in obese and weight-losing individuals. The Journal of Clinical Endocrinology & Metabolism. 2002;87(4):1708–16.
Faraj M, Havel PJ, Phélis S, et al. Plasma acylation-stimulating protein, adiponectin, leptin, and ghrelin before and after weight loss induced by gastric bypass surgery in morbidly obese subjects. The Journal of Clinical Endocrinology & Metabolism. 2003;88(4):1594–602.
Camastra S, Manco M, Frascerra S, et al. Daylong pituitary hormones in morbid obesity: effects of bariatric surgery. Int J Obes. 2009;33(1):166.
Cummings DE, Weigle DS, Frayo RS, et al. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N Engl J Med. 2002;346(21):1623–30.
Cugno M, Castelli R, Mari D, et al. Inflammatory and prothrombotic parameters in normotensive non-diabetic obese women: effect of weight loss obtained by gastric banding. Intern Emerg Med. 2012;7(3):237–42.
Eržen B, Šabovič M. In young post-myocardial infarction male patients elevated plasminogen activator inhibitor-1 correlates with insulin resistance and endothelial dysfunction. Heart Vessel. 2013;28(5):570–7.
Piestrzeniewicz K, Łuczak K, Goch JH. Factors associated with C-reactive protein at the early stage of acute myocardial infarction in men. Cardiology journal. 2009;16(1):36–42.
Martin SS, Qasim A, Reilly MP. Leptin resistance: a possible interface of inflammation and metabolism in obesity-related cardiovascular disease. J Am Coll Cardiol. 2008;52(15):1201–10.
Messier V, Karelis AD, Prud'homme D, et al. Identifying metabolically healthy but obese individuals in sedentary postmenopausal women. Obesity. 2010;18(5):911–7.
Iyer A, Fairlie DP, Prins JB, et al. Inflammatory lipid mediators in adipocyte function and obesity. Nat Rev Endocrinol. 2010;6(2):71.
Netto BDM, Bettini SC, Clemente APG, et al. Roux-en-Y gastric bypass decreases pro-inflammatory and thrombotic biomarkers in individuals with extreme obesity. Obes Surg. 2015;25(6):1010–8.
Weyer C, Funahashi T, Tanaka S, et al. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. The Journal of Clinical Endocrinology & Metabolism. 2001;86(5):1930–5.
Stefan N, Vozarova B, Funahashi T, et al. Plasma adiponectin concentration is associated with skeletal muscle insulin receptor tyrosine phosphorylation, and low plasma concentration precedes a decrease in whole-body insulin sensitivity in humans. Diabetes. 2002;51(6):1884–8.
Papailiou J, Albanopoulos K, Toutouzas KG, et al. Morbid obesity and sleeve gastrectomy: how does it work? Obes Surg. 2010;20(10):1448–55.
Zhou D, Jiang X, Ding W, et al. Impact of bariatric surgery on ghrelin and obestatin levels in obesity or type 2 diabetes mellitus rat model. Journal of diabetes research. 2014;2014
Davenport AP, Bonner TI, Foord SM, et al. International Union of Pharmacology. LVI. Ghrelin receptor nomenclature, distribution, and function. Pharmacol Rev. 2005;57(4):541–6.
Murphy KG, Dhillo WS, Bloom SR. Gut peptides in the regulation of food intake and energy homeostasis. Endocr Rev. 2006;27(7):719–27.
Chronaiou A, Tsoli M, Kehagias I, et al. Lower ghrelin levels and exaggerated postprandial peptide-YY, glucagon-like peptide-1, and insulin responses, after gastric fundus resection, in patients undergoing Roux-en-Y gastric bypass: a randomized clinical trial. Obes Surg. 2012;22(11):1761–70.
le Roux CW, Welbourn R, Werling M, et al. Gut hormones as mediators of appetite and weight loss after Roux-en-Y gastric bypass. Ann Surg. 2007;246(5):780–5.
Rodieux F, Giusti V, D'alessio DA, et al. Effects of gastric bypass and gastric banding on glucose kinetics and gut hormone release. Obesity. 2008;16(2):298–305.
Laferrère B, Teixeira J, McGinty J, et al. Effect of weight loss by gastric bypass surgery versus hypocaloric diet on glucose and incretin levels in patients with type 2 diabetes. The Journal of Clinical Endocrinology & Metabolism. 2008;93(7):2479–85.
Peterli R, Steinert RE, Woelnerhanssen B, et al. Metabolic and hormonal changes after laparoscopic Roux-en-Y gastric bypass and sleeve gastrectomy: a randomized, prospective trial. Obes Surg. 2012;22(5):740–8.
Saltiel AR, Olefsky JM. Inflammatory mechanisms linking obesity and metabolic disease. J Clin Invest. 2017;127(1):1–4.
Illán Gómez F, Gonzálvez Ortega M, Aragón Alonso A, et al. Obesidad, inflamación y función endotelial: efectos de la pérdida de peso tras cirugía bariatrica. Nutr Hosp. 2016;33(6):1340–6.
Forsythe LK, Wallace JM, Livingstone MBE. Obesity and inflammation: the effects of weight loss. Nutr Res Rev. 2008;21(2):117–33.
Hagman DK, Larson I, Kuzma JN, et al. The short-term and long-term effects of bariatric/metabolic surgery on subcutaneous adipose tissue inflammation in humans. Metabolism-Clinical and Experimental. 2017;70:12–22.
Mottaghi A, Mirmiran P, Delshad H, et al. Effect of different obesity phenotypes on incidence of chronic kidney disease in Tehranian adults. J Am Coll Nutr. 2016;35(7):587–96.
Dandona P, Weinstock R, Thusu K, et al. Tumor necrosis factor-α in sera of obese patients: fall with weight loss. The Journal of Clinical Endocrinology & Metabolism. 1998;83(8):2907–10.
Laimer M, Ebenbichler C, Kaser S, et al. Markers of chronic inflammation and obesity: a prospective study on the reversibility of this association in middle-aged women undergoing weight loss by surgical intervention. Int J Obes. 2002;26(5):659.
Kopp H-P, Kopp C, Festa A, et al. Impact of weight loss on inflammatory proteins and their association with the insulin resistance syndrome in morbidly obese patients. Arterioscler Thromb Vasc Biol. 2003;23(6):1042–7.
Dandona P, Aljada A, Mohanty P, et al. Insulin inhibits intranuclear nuclear factor κB and stimulates IκB in mononuclear cells in obese subjects: evidence for an anti-inflammatory effect? The Journal of Clinical Endocrinology & Metabolism. 2001;86(7):3257–65.
Davì G, Guagnano MT, Ciabattoni G, et al. Platelet activation in obese women: role of inflammation and oxidant stress. JAMA. 2002;288(16):2008–14.
Tripathy D, Mohanty P, Dhindsa S, et al. Elevation of free fatty acids induces inflammation and impairs vascular reactivity in healthy subjects. Diabetes. 2003;52(12):2882–7.
Jiménez A, Casamitjana R, Flores L, et al. Long-term effects of sleeve gastrectomy and Roux-en-Y gastric bypass surgery on type 2 diabetes mellitus in morbidly obese subjects. Ann Surg. 2012;256(6):1023–9.
Morínigo R, Casamitjana R, Delgado S, et al. Insulin resistance, inflammation, and the metabolic syndrome following Roux-en-Y gastric bypass surgery in severely obese subjects. Diabetes Care. 2007;30(7):1906–8.
Arismendi E, Rivas E, Agustí A, et al. The systemic inflammome of severe obesity before and after bariatric surgery. PLoS One. 2014;9(9):e107859.
Cancello R, Henegar C, Viguerie N, et al. Reduction of macrophage infiltration and chemoattractant gene expression changes in white adipose tissue of morbidly obese subjects after surgery-induced weight loss. Diabetes. 2005;54(8):2277–86.
Flevaris P, Vaughan D, editors. The role of plasminogen activator inhibitor type-1 in fibrosis. Seminars in thrombosis and hemostasis; 2017: Thieme Medical Publishers.
Khan SS, Lloyd-Jones DM, Chan C, Liu K, Cushman M, Kestenbaum B, et al. Association of plasminogen activator inhibitor-1 with prevalent and incident obesity is independent of inflammatory markers: the Multi-Ethnic Study of Atherosclerosis (MESA). Am Heart Assoc; 2015.
Alessi M, Peiretti F, Morange P, et al. Production of plasminogen activator inhibitor 1 by human adipose tissue: possible link between visceral fat accumulation and vascular disease. Diabetes. 1997;46(5):860–7.
Tschoner A, Sturm W, Engl J, et al. Plasminogen activator inhibitor 1 and visceral obesity during pronounced weight loss after bariatric surgery. Nutrition, metabolism, and cardiovascular diseases : NMCD. 2012;22(4):340–6.
Morel O, Luca F, Grunebaum L, Jesel L, Meyer N, Desprez D, et al. Short-term very low-calorie diet in obese females improves the haemostatic balance through the reduction of leptin levels, PAI-1 concentrations and a diminished release of platelet and leukocyte-derived microparticles. International journal of obesity (2005). 2011 Dec;35(12):1479–86.
Bosanská L, Michalský D, Lacinová Z, et al. The influence of obesity and different fat depots on adipose tissue gene expression and protein levels of cell adhesion molecules. Physiol Res. 2010;59(1):79.
Ziccardi P, Nappo F, Giugliano G, et al. Reduction of inflammatory cytokine concentrations and improvement of endothelial functions in obese women after weight loss over one year. Circulation. 2002;105(7):804–9.
Vázquez LA, Pazos F, Berrazueta JR, et al. Effects of changes in body weight and insulin resistance on inflammation and endothelial function in morbid obesity after bariatric surgery. The Journal of Clinical Endocrinology & Metabolism. 2005;90(1):316–22.
Acknowledgements
Research reported in this publication was supported by the Elite Researcher Grant Committee under award number 958714 from the National Institutes for Medical Research Development (NIMAD), Tehran, Iran.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Author 2 reports grants from National Institutes for Medical Research Development (NIMAD), during the conduct of the study. All of the other authors report no conflict of interest.
Informed Consent Statement
Informed consent does not apply.
Ethical Approval Statement
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Khosravi-Largani, M., Nojomi, M., Aghili, R. et al. Evaluation of all Types of Metabolic Bariatric Surgery and its Consequences: a Systematic Review and Meta-Analysis. OBES SURG 29, 651–690 (2019). https://doi.org/10.1007/s11695-018-3550-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-018-3550-z