Abstract
Background
This study aims to quantify changes in fibroblast growth factor 19 (FGF19) and bile acids (BAs) in patients with uncontrolled type 2 diabetes randomized to Roux-en-Y gastric bypass (RYGB) vs intensive medical management (IMM) and matched for similar reduction in HbA1c after 1 year of treatment.
Methods
Blood samples were drawn from patients who underwent a test meal challenge before and 1 year after IMM (n = 15) or RYGB (n = 15).
Results
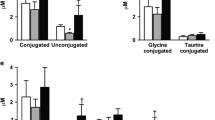
Mean HbA1c decreased from 9.7 to 6.4 % after RYGB and from 9.1 to 6.1 % in the IMM group. At 12 months, the number of diabetes medications used per subject in the RYGB group (2.5 ± 0.5) was less than in the IMM group (4.6 ± 0.3). After RYGB, FGF19 increased in the fasted (93 ± 15 to 152 ± 19 pg/ml; P = 0.008) and postprandial states (area under the curve (AUC), 10.8 ± 1.9 to 23.4 ± 4.1 pg × h/ml × 103; P = 0.006) but remained unchanged following IMM. BAs increased after RYGB (AUC ×103, 6.63 ± 1.3 to 15.16 ± 2.56 μM × h; P = 0.003) and decreased after IMM (AUC ×103, 8.22 ± 1.24 to 5.70 ± 0.70; P = 0.01). No changes were observed in the ratio of 12α-hydroxylated/non-12α-hyroxylated BAs. Following RYGB, FGF19 AUC correlated with BAs (r = 0.54, P = 0.04) and trended negatively with HbA1c (r = −0.44; P = 0.09); these associations were not observed after IMM.
Conclusions
BA and FGF19 levels increased after RYGB but not after IMM in subjects who achieved similar improvement in glycemic control. Further studies are necessary to determine whether these hormonal changes facilitate improved glucose homeostasis.



Similar content being viewed by others
References
Brolin RE. Bariatric surgery and long-term control of morbid obesity. JAMA. 2002;288(22):2793–6.
Dixon JB, Zimmet P, Alberti KG, et al. Bariatric surgery: an IDF statement for obese type 2 diabetes. Diabet Med. 2011;28(6):628–42.
American Diabetes Association. Approaches to glycemic treatment. Diabetes Care. 2015; 38 Suppl:S41-8.
Korner J, Bessler M, Cirilo LJ, et al. Effects of Roux-en-Y gastric bypass surgery on fasting and postprandial concentrations of plasma ghrelin, peptide YY, and insulin. J Clin Endocrinol Metab. 2005;90(1):359–65.
Korner J, Inabnet W, Conwell IM, et al. Differential effects of gastric bypass and banding on circulating gut hormone and leptin levels. Obesity. 2006;14(9):1553–61.
Korner J, Inabnet W, Febres G, et al. Prospective study of gut hormone and metabolic changes after adjustable gastric banding and Roux-en-Y gastric bypass. Int J Obes. 2009;33(7):786–95.
Nguyen KT, Korner J. The sum of many parts: potential mechanisms for improvement in glucose homeostasis after bariatric surgery. Curr Diab Rep. 2014;14(5):481.
Nguyen NQ, Debreceni TL, Bambrick JE, et al. Rapid gastric and intestinal transit is a major determinant of changes in blood glucose, intestinal hormones, glucose absorption and postprandial symptoms after gastric bypass. Obesity (Silver Spring). 2014;22(9):2003–9.
Inagaki T, Choi M, Moschetta A, et al. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab. 2005;2(4):217–25.
Choi M, Moschetta A, Bookout AL, et al. Identification of a hormonal basis for gallbladder filling. Nat Med. 2006;12(11):1253–5.
Kir S, Beddow SA, Samuel VT, et al. FGF19 as a postprandial, insulin-independent activator of hepatic protein and glycogen synthesis. Science. 2011;331(6024):1621–4.
Kir S, Kliewer S, Mangelsdorf D. Roles of FGF19 in liver metabolism. Cold Spring Harbor Symp Quant Biol. 2011;LXXVI:1–6.
Wu AL, Coulter S, Liddle C, et al. FGF19 regulates cell proliferation, glucose and bile acid metabolism via FGFR4-dependent and independent pathways. PLoS One. 2011;6(3), e17868.
Fu L, John LM, Adams SH, et al. Fibroblast growth factor 19 increases metabolic rate and reverses dietary and leptin-deficient diabetes. Endocrinology. 2004;145(6):2594–603.
Tomlinson E, Fu L, John L, et al. Transgenic mice expressing human fibroblast growth factor-19 display increased metabolic rate and decreased adiposity. Endocrinology. 2002;143(5):1741–7.
Ryan KK, Tremaroli V, Clemmensen C, et al. FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nature. 2014;509(7499):183–8.
Watanabe M, Houten SM, Wang L, et al. Bile acids lower triglyceride levels via a pathway involving FXR, SHP, and SREBP-1c. J Clin Invest. 2004;113(10):1408–18.
Watanabe M, Houten SM, Mataki C, et al. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature. 2006;439(7075):484–9.
Katsuma S, Hirasawa A, Tsujimoto G. Bile acids promote glucagon-like peptide-1 secretion through TGR5 in a murine enteroendocrine cell line STC-1. Biochem Biophys Res Commun. 2005;329(1):386–90.
Thomas C, Gioiello A, Noriega L, et al. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab. 2009;10(3):167–77.
Modica S, Gadaleta RM, Moschetta A. Deciphering the nuclear bile acid receptor FXR paradigm. Nuclear Recept Signal. 2010;8, e005.
Parks DJ, Blanchard SG, Bledsoe RK, et al. Bile acids: natural ligands for an orphan nuclear receptor. Science. 1999;284(5418):1365–8.
Haeusler RA, Astiarraga B, Camastra S, et al. Human insulin resistance is associated with increased plasma levels of 12alpha-hydroxylated bile acids. Diabetes. 2013;62(12):4184–91.
Patti ME, Houten SM, Bianco AC, et al. Serum bile acids are higher in humans with prior gastric bypass: potential contribution to improved glucose and lipid metabolism. Obesity (Silver Spring Md). 2009;17(9):1671–7.
Nakatani H, Kasama K, Oshiro T, et al. Serum bile acid along with plasma incretins and serum high-molecular weight adiponectin levels are increased after bariatric surgery. Metabolism. 2009;58(10):1400–7.
Jansen PL, van Werven J, Aarts E, et al. Alterations of hormonally active fibroblast growth factors after Roux-en-Y gastric bypass surgery. Dig Dis. 2011;29(1):48–51.
Pournaras DJ, Glicksman C, Vincent RP, et al. The role of bile after Roux-en-Y gastric bypass in promoting weight loss and improving glycaemic control. Endocrinology. 2012;153(8):3613–9.
Simonen M, Dali-Youcef N, Kaminska D, et al. Conjugated bile acids associate with altered rates of glucose and lipid oxidation after Roux-en-Y gastric bypass. Obes Surg. 2012;22(9):1473–80.
Ahmad NN, Pfalzer A, Kaplan LM. Roux-en-Y gastric bypass normalizes the blunted postprandial bile acid excursion associated with obesity. Int J Obes (2005). 2013;37(12):1553–9.
Kohli R, Bradley D, Setchell KD, et al. Weight loss induced by Roux-en-Y gastric bypass but not laparoscopic adjustable gastric banding increases circulating bile acids. J Clin Endocrinol Metab. 2013;98(4):E708–12.
Gerhard GS, Styer AM, Wood GC, et al. A role for fibroblast growth factor 19 and bile acids in diabetes remission after Roux-en-Y gastric bypass. Diabetes Care. 2013;36(7):1859–64.
Ikramuddin S, Korner J, Lee WJ, et al. Roux-en-Y gastric bypass vs intensive medical management for the control of type 2 diabetes, hypertension, and hyperlipidemia: the Diabetes Surgery Study randomized clinical trial. JAMA. 2013;309(21):2240–9.
Thomas AJ, Bainbridge HA, Schone JL, et al. Recruitment and screening for a randomized trial investigating Roux-en-Y gastric bypass versus intensive medical management for treatment of type 2 diabetes. Obes Surg. 2014;24(11):1875–80.
Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–9.
Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22(9):1462–70.
Wewalka M, Patti ME, Barbato C, et al. Fasting serum taurine-conjugated bile acids are elevated in type 2 diabetes and do not change with intensification of insulin. J Clin Endocrinol Metab. 2014;99(4):1442–51.
Kohli R, Kirby M, Setchell KD, et al. Intestinal adaptation after ileal interposition surgery increases bile acid recycling and protects against obesity-related comorbidities. Am J Physiol Gastrointest Liver Physiol. 2010;299(3):G652–60.
Furet JP, Kong LC, Tap J, et al. Differential adaptation of human gut microbiota to bariatric surgery-induced weight loss: links with metabolic and low-grade inflammation markers. Diabetes. 2010;59(12):3049–57.
Steinert RE, Peterli R, Keller S, et al. Bile acids and gut peptide secretion after bariatric surgery: a 1-year prospective randomized pilot trial. Obesity (Silver Spring). 2013;21(12):E660–8.
Morton GJ, Kaiyala KJ, Foster-Schubert KE, et al. Carbohydrate feeding dissociates the postprandial FGF19 response from circulating bile acid levels in humans. J Clin Endocrinol Metab. 2014;99(2):E241–5.
Meyer-Gerspach AC, Steinert RE, Keller S, et al. Effects of chenodeoxycholic acid on the secretion of gut peptides and fibroblast growth factors in healthy humans. J Clin Endocrinol Metab. 2013;98(8):3351–8.
Acknowledgments
The investigators express gratitude to all study participants and recognize the contributions of the study coordinators, Joyce Schone, RD and Nyra Wimmergren, RN (University of Minnesota), Heather Bainbridge RD, CDN (Columbia University Medical Center), Avis Thomas, MS (statistician, University of Minnesota Data Coordinating Center), and Irene M. Conwell and Tiffany Thomas, Ph.D. (Columbia University Medical Center) for expert technical assistance.
Conflict of Interest
All authors have completed and submitted Disclosure of Potential Conflicts of Interest. Dr Billington reports receiving grant support from Covidien and personal support for consultancy from EnteroMedics Inc. Dr Connett reports receiving grant support from Covidien. Dr Ikramuddin serves as an advisory board member for Novo Nordisk, USGI, and Medica; consults for Metamodix Inc; and receives grant support from Covidien, EnteroMedics, and ReShape Medical. Dr Korner reports receiving institutional grant support from Covidien, travel expenses from American Board of Obesity Medicine, and personal support for serving on the Takeda Speaker Bureau and on the Scientific Advisory Board for Nutrisystem and consulting for the Office of Professional Misconduct and L.E.K Consulting.
Ethical Approval
All procedures involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Funding
The study was supported in part by Covidien, Mansfield, MA. The sponsoring agency had no role in the collection, management, analysis, and interpretation of the study data and had no part in the preparation of the manuscript. The sponsor was allowed to review the manuscript prior to submission but had no role in the decision to submit the manuscript for publication. Additional support was received from National Institutes of Health grant DK072011 (J.K.), the National Center for Advancing Translations Sciences Grant UL1 TR000040, and discretionary funds from Columbia University Medical Center (to J.K.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sachdev, S., Wang, Q., Billington, C. et al. FGF 19 and Bile Acids Increase Following Roux-en-Y Gastric Bypass but Not After Medical Management in Patients with Type 2 Diabetes. OBES SURG 26, 957–965 (2016). https://doi.org/10.1007/s11695-015-1834-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-015-1834-0