Abstract
The interplay between ecological diversification and sexual dimorphism has been largely overlooked in the literature. Sexually dimorphic species which are also undergoing adaptive radiations are ideal for filling this knowledge gap. The Arctic charr in lake Thingvallavatn is one such system: it is a sexually dimorphic species which has recently diverged along the benthic-limnetic ecological axis. In a long-running common-garden experiment we studied the shape variation throughout ontogeny of intra- and inter- morph crosses of benthic and limnetic charr from the lake. We found that shape differences between ecomorphs and sexes had a genetic component. Prior to the onset of sexual maturation, shape differences were attributable to cross type and were related to adaptations to benthic and limnetic niches, i.e., shorter lower jaws and rounder snouts in the benthic and evenly protruding snouts and pointier snouts in the limnetic. Reciprocal hybrids showed intermediate, transgressive and/or maternal morphologies. However, after the onset of sexual maturation larger morphological differences occurred between sexes than among cross types. Taken together, our results demonstrate that the interplay between ecological diversification and sexual dimorphism is complex and dynamic throughout ontogeny, and that long-term common garden experiments are immensely valuable for studying shape dynamics in different evolutionary scenarios.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Adaptive radiations provide unique insights into the origins of polymorphic populations (Losos, 2010; Schluter, 2000). Often, species undergoing adaptive divergence also exhibit sexual dimorphism (Gillespie, 2004; Grant & Grant, 2002; Jones et al., 2012; Losos & Schneider, 2009; Meyer, 1993; Snorrason & Skúlason, 2004). However, the interplay between traits related to adaptive divergence and secondary sexual traits has been largely overlooked (but see Bolnick & Doebeli, 2003; Butler et al., 2007; Berra, 2001; Cooper et al., 2011).
Resource polymorphism is a major driver of adaptive divergence where organisms exploit different resources, leading to phenotypic and/or genotypic differentiation (Skúlason et al., 1993; Smith & Skúlason, 1996). In teleosts, common cases of resource polymorphism occur along benthic-limnetic ecological axes (Seehausen & Wagner, 2014). Phenotypes associated with such divergence are often adaptive: fish occupying benthic ecological niches usually have subterminal jaws, blunt snouts, deep bodies, smaller eyes and less gill rackers, whereas fish occupying the limnetic zone have terminal jaws, pointed snouts, slender bodies, bigger eyes and more gill rackers (Berra, 2001; Blake et al., 2005; Hulsey et al., 2013; Sandlund et al., 1992; Snorrason et al., 1994). In recent adaptive radiations, the interplay between traits associated with benthic-limnetic adaptations and sexual dimorphism is best studied in sticklebacks (e.g,:Aguirre et al., 2008; Berner et al., 2010; Cooper et al., 2011; Kitano et al., 2007; McGee & Wainwright, 2013; Reimchen & Nosil, 2004). Although very few examples exist in salmonids (Bjoeru & Sandlund, 1995; Janhunen et al., 2009) these studies already paint a complex and dynamic picture of sexual dimorphism throughout the lifecycle.
Recently diverged populations with relatively simple demographic histories and that are still hybridising facilitate the study of the genetic basis of adaptive traits. Hybridisation between or within closely related species will lead to a breakdown of coadapted alleles, heterosis and/or non-additive effects (Dobzhansky, 1936; Ackermann et al., 2006; Arnegard et al., 2014), and see (Bell and Travis, 2005) which will result in intermediate, transgressive and/or parental-like traits (e.g., Seehausen, 2004; Elgvin et al., 2017; Skúlason et al., 1989, respectively). Yet, other phenomena such as genotype x environment interactions and phenotypic plasticity can have strong confounding effects. Despite their logistic inconveniences, common garden experiments are a conceptually straightforward solution to overcome these effects, as they standarise for environmental cues (Villemereuil et al., 2016). Moreover, long term common garden experiments will allow us to gain insight into how the genetic component of morphological traits unfolds throughout ontogeny (e.g., Vučić et al., 2019).
In the present study, we used a long-running common garden setup, encompassing the onset of sexual maturation, to determine the factors responsible for the genetically based shape variation during the post-embryonic ontogeny of a textbook example of a polymorphic species: the Arctic charr in lake Thingvallavatn (Iceland). In Thingvallavatn, Arctic charr has diverged along the ecological benthic-limnetic axis in four ecomorphs (two benthic and two limnetic). For this study, we focused on one benthic (small benthic, SB) and one limnetic (planktivorous, PL) morph, with strong phenotypic differences. Despite recent evolutionary divergence, their overlapping spawning periods (Jonsson et al., 1988) and comparable sexual maturation times (Snorrason et al., 1989), gene flow between SB and PL is rather low (Brachmann et al., 2021; Jonsson et al., 1988; Kapralova et al., 2011; Skúlason et al., 1989), but it is still possible to generate hybrids in captivity. Offspring of wild SB and PL morphs from the lake and their reciprocal hybrids were reared in common garden up to 36 months and phenotyped at four time points during ontogeny.
The aim of this study was to investigate the factors driving genetically based shape variation throughout the ontogeny of two Arctic charr ecomorphs and their hybrids. We hypothesized that (1) traits associated with benthic-limnetic adaptations will have a non-additive genetic effect, (2) the effective load of morphs will increase during ontogeny, and (3) sexually dimorphic traits will only be detectable after the onset of sexual maturation.
Material and Methods
Study System
Thingvallavatn is a post-glacial lake situated in south-western Iceland, colonised by Arctic charr (Salvelinus alpinus) less than 12 K years ago. After this single colonisation event, Arctic charr diverged into four ecomorphs along the benthic-limnetic axis, representing a text-book case of resource polymorphism. The diverged morphs resulted in two bottom-feeders (i.e., a small and a large benthic, SB and LB) and two limnetic-feeders (i.e., a planktivorous and a piscivorous morph, PL and PI) (Guðbrandsson et al., 2018; Jonsson et al., 1988; Kapralova et al., 2011, 2013; Malmquist et al., 1992; Sandlund et al., 1992; Snorrason et al., 1989).
Traits associated with benthic-limnetic adaptations also occur throughout ontogeny in the form of an ontogenetic shift. All Arctic charr ecomorphs start feeding on the benthos at juvenile stages (Parsons et al., 2011; Skúlason et al., 1989), and limnetic morphs migrate towards pelagic areas in order to feed on plankton (planktivorous morph, PL) or other fish (piscivorous, PI), whilst benthic morphs retain these juvenile-like traits to continue feeding on the benthos (Skúlason et al., 1989).
Data Collection
Generation of Crosses and Rearing
Wild adult specimens of the planktivorous (PL) and small benthic (SB) morphs were collected by laying gillnets overnight at a spawning site shared by both morphs (Svínanesvík, 64°11′24.6"N; 21°05′40.5"W) in the beginning of October 2015. A total of 31 mature specimens were crossed either within the same morph (i.e., intra-morph crosses, PLxPL and SBxSB) or reciprocally with the alternative morph (i.e., reciprocal hybrid crosses, PLxSB and SBxPL, always ♀ x ♂), generating 19 full-sibling families (see crossing design in Supplementary material (SM), table S1). Fertilised eggs from each family were placed in separate mesh cages in an EWOS hatching tray (EWOS, Norway) at 4.1 ± 0.2 °C in the aquaculture facilities of Hólar University College, Sauðárkrókur, Iceland. After hatching and first feeding, families were transferred into separate buckets (35 cm deep, ∅ 29 cm), connected to the same running water flow and fed manually with commercial dry pellets in the same way to ensure common feeding conditions. Fish were phenotyped throughout ontogeny at 12, 18, 24 and 36 months after fertilisation. At each time point, specimens were anaesthetized with appropriate dose of 2-phenoxyethanol (Pounder et al., 2018). At 12 months specimens were only photographed and no individual information was collected. Those families with a larger number of individuals were separated into two buckets to control for density. At 18 months all fish were PIT- tagged, weighted, photographed and placed together in two aquaculture tanks with similar densities. At month 24, fish were again weighted and photographed, and at month 36 they were photographed, and their sex determined if they had reached sexual maturation. Since fish were PIT- tagged at month 18, individual information collected at later time points could be traced back. However, no sex data were available for month 12. Mortality throughout the experimental setup was low (less than 10%), and higher levels of mortality were not attributed to any specific cross type or family.
Photographing
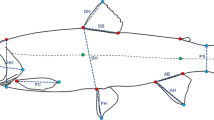
Each individual was photographed at 12, 18, 24 and 36 months. Photos were taken on their left lateral side with a fixed digital camera (Canon EOS 650D and 100 mm macro lens) along with a ruler for scaling. Fish tend to naturally bend as they are positioned on a rigid flat surface while the photos are being taken, which may confound biologically meaningful shape variation (Valentin et al., 2008). We corrected for potential bending effects by placing 5 equidistant landmarks along the lateral line of each fish and implementing the unbending tool in tpsUtil (Rohlf & tpsDig, 2016).
Landmarking
We placed 16 landmarks and 4 semilandmarks on each photo using tpsDig (version 2.26, (Rohlf & tpsDig, 2016)) (Fig. 1), following (Adams & Huntingford, 2004; Parsons et al., 2010) and see landmark description in SM2. For scaling, two additional landmarks were placed on a ruler and were removed before performing Procrustes superimposition. Landmarking of the data was conducted by the same person. Thirty random individuals from different cross types and families were landmarked three times, obtaining a high repeatability (p < 0.05) which ensured the robustness of the data.
Analysis of Shape
Raw coordinates in.tps format were imported in R and subsequent analyses were conducted with the geometric morphometrics package geomorph v4.0.0 and RRPP v1.0.0 (Baken et al., 2021; Collyer & Adams, 2018, 2021). Outlier examination was conducted by looking at Procrustes distance of each landmark configuration to their mean shape, grouped by month and cross type. Configurations falling on the upper quantile of the distribution represented 2.18% of the full data set and were inspected individually. These few specimens showed either landmark displacements or slightly open jaws, were considered outliers and were removed from the final dataset. After outlier removal, Partial Generalized Procrustes Superimposition was performed on raw data and the resulting Procrustes coordinates were used in downstream analyses (see Table 1 for number of individuals used at each time point). During Procrustes superimposition, each landmark configuration is translated, scaled and rotated to minimise shape differences among them.
Centroid size (the square root of the sum of squared distances of the landmarks to the centroid) is extracted during scaling and it is often used as a proxy for body size (e.g., Parsons et al., 2010; Kapralova et al., 2015). This may not be the case for our dataset as Arctic charr have fusiform bodies and we unevenly positioned the landmarks along the shape outline (a considerable proportion of the landmarks were on their heads) (Collyer et al., 2020). As body weight information was missing for months 12 and 36, we tested whether centroid size could be used as a reliable indicator for body weight for our dataset. A high correlation between centroid size and body weight (98.6% (Pearson’s correlation coefficient, p < 0.05) on the log–log regression of both variables) for a subset of individuals (N18 months = 653, N24 months = 595) confirmed that centroid size can be used as a proxy for total body size in our study.
Statistical analyses were performed at two different levels: (1) on a full data set of specimens to study the dynamic patterns of shape variation across four time points (i.e., month 12, 18, 24 and 36) with no sex information and hence heterogeneity in the sexual maturation state of the specimens and (2) on specimens which reached sexual maturation during the experimental setup and thus individual sex information could be traced back to month 18 and studied across time. For both datasets, mixed-model MANOVAs were performed with geomorph::procD.lm to examine the effects of log(Csize), month, sex (if applicable) and cross type with nested families (cross/family) (including all possible interactions) on shape (i.e., and here after, Procrustes coordinates). The RRPP::pairwise function was used to assess differences in shape between group means and variances (the latter as a proxy for morphological disparity). These analyses were performed at each separate time point and also pooling together individuals phenotyped at different time points. To study ontogenetic trajectories of sexual dimorphism (months 18, 24 and 36) we performed Phenotypic Trajectory Analysis (PTA) in geomorph. Due to unbalanced number of sexed individuals among cross types and families (see Table 1, and SM1 Table 1), we decided to pool the four cross types together and study intraspecific sex trajectories as a whole. We explored pairwise differences in length (i.e., amount of shape change), directionality, shape and location in the morphospace between both trajectories. For the same data set, MANOVAs and pairwise tests were performed separately on the different time points. We used geomorph::plotAllometry for visual interpretation of static allometry patterns and Homogeneity of Slopes (HOS) tests were conducted by comparing models of common (shape ~ log(Csize) + sex) versus unique allometry (shape ~ log(Csize) * sex) statistically in order to decide whether to correct for static allometric effects within each time point.
Visualisation
Principal Component Analyses (PCA) on Procrustes coordinates (Adams et al., 2013) were conducted for visualisation of shape variation at each level. Additionally, we used wireframes to explore shape changes in landmark configurations at the extremes of the most relevant eigenvectors (Bookstein, 1989). Ontogenetic trajectories were plotted onto the first two principal components based on the covariance matrix of group means. Trajectories connect mean shape estimates at the different time points for each sex or cross type.
Results
Benthic and Limnetic Traits Have a Genetic Component
We found significant differences in mean shape between PLxPL and SBxSB within each time point (p < 0.05, see mean pairwise comparisons in SM4) when fitting explicit reduced models (shape ~ 1 + log(Csize)). However, we did not see significant differences in shape variance (SM4).
Except for month 36, either the first principal component, the second or both separate the PLxPL and SBxSB crosses (Fig. 2). Wireframes representing the predicted shapes at the extremes of those PCs are consistent among each other: intramorph SB crosses tend to have deeper bodies, deeper heads and rounder snouts compared to intramorph PL crosses, which have more fusiform bodies, narrower heads and pointier snouts. However, the position of the reciprocal hybrids in the morphospace is not as consistent, indicating that their shape variation is highly dynamic throughout ontogeny. Specifically, at month 12 (Fig. 2A) reciprocal hybrids laid in the middle, but they did not completely overlap with each other, being the maternal morph closer to each of them. At month 18 (Fig. 2B), a substantial proportion of hybrids showed transgressive (i.e., outside of the parental range) phenotypes at high values of PC1, the main axis of variance (29.95%). The rest of the hybrid specimens at month 18 laid between their parental phenotypes. At month 24 (Fig. 2C) the pattern again switched, showing that hybrid shape variation was mostly contained within their maternal morphospace. Nevertheless, the positioning of reciprocal hybrids was not symmetrical: SBxPL shape variability is particularly reduced compared to PLxSB, which completely overlapped with PLxPL, and also expanded towards SBxSB and SBxPL’s morphospaces.
Two first principal componensts of separate principal component analyses of months 12 (A), 18 (B), 24 (C) and 36 (D). Each point represents one individual and shaded areas depict 95% confidence ellipses. Grids represent the predicted mean shape of each cross. The observed shape changes in the grids are in respect to the grand mean of all individuals per time point. Two morphs are represented by one grid when their mean shapes were not significantly different from the grand mean
Although cross type was found to have a significant effect on shape at month 36 (Fig. 3) (and overall shape differences were significant between all pairs (SM4, table S4.10)), no clear cross type clustering was found when examining the first ten principal components (Fig. 2D).
Regarding allometry, only in month 12 the null hypothesis of homogeneity of slopes between cross types was not rejected (p = 1.000). However, we decided not to correct for common allometry not only for easier comparison among time points, but also because shape variation due to change plays an important role during ontogeny.
Sexual Dimorphism is Present Before Sexual Maturation Occurs
When looking at the reduced dataset that includes sex, the effect of sex alone on shape was significant both when all the individuals from different time points are pooled together (SM3) and at each separate time point, although the effect was particularly important at months 24 and 36 (Fig. 3, SM3).
We focused on sexually mature individuals at month 36 to study the maximum dissimilarity in shape between males and females. The shape variation along PC1 (Fig. 4) was explained by differences between sexes, with certain point overlap between the two clusters. The wireframes depicted that females had substantially smaller heads compared to males, more stream-lined bodies and slightly longer caudal peduncles. Compared to females, males seemed to have pointier snouts, but no differences in the position of upper and lower jaw were detected. However, we were not able to determine to what extent traits associated with sexual dimorphism are confounded due to the effect of traits associated to benthic-limnetic adaptations, which do not lay at PC extremes and thus their variation cannot be represented in a PCA. Despite the fact that it was not possible to study sexual dimorphism within cross type due to data imbalance (especially in the case of the SB crosses), we examined whether intra-morph crosses or reciprocal hybrids clustered towards the “most male or female” morphospace, but found no clear structure (SM6 for visualisation). Another confounding factor may be the heterogeneous sexual maturation time (i.e., some individuals were not mature yet, and thus not sexed and not used for sexual dimorphism analysis) and/or other differences in life history traits between morphs (Jonsson et al., 1988) that would affect shape.
Complex Interplay Between Benthic-Limnetic Traits and Secondary Sexual Traits
Despite both cross and sex being significant across ontogeny, these terms showed opposite tendencies (Fig. 3): while the effect of sex increased, cross, which had larger load than sex at month 18, greatly decreased towards month 36. We found that males were significantly larger than females at months 18 and 24 (t = 3.134, p = 0.0018 and t = 2.29, p = 0.0013 respectively), but no centroid size differences were found at month 36 (t = −1.479, p = 0.14; see SM7), suggesting that either the effect of size on the expression of sexual dimorphism is negligible or that it is dynamic, and its signal is diluted by other confounding factors.
We then asked whether ontogenetic trajectories between the two morphs differed and what the position of the hybrids was. Phenotypic trajectory analysis (PTA) showed overall differences in ontogenetic trajectories between each cross type (Fig. 5A). We found significant differences in trajectory location between all pairs (p = 0.001), direction (Pr angle ≤ 0.007 for each pair), and shape (Pr>d = 0.001 for each pair). However, only significant differences in the amount of shape change (i.e., absolute path trajectory lengths) were found between both intra-morph crosses and PLxSB (Pr>d = 0.007 and 0.004 respectively). Of the four cross types, PLxSB appeared to have the longest trajectory (maximum absolute path distance = 0.0672) (Fig. 5A). At month 36, cross type means converged in both PC1 and PC2, although morphological disparity at this time point increased, likely driven by -at least- individuals who reached sexual maturation and showed secondary sexual traits (SM8).
Phenotypic Trajectory Analysis onto the first two principal components based on the covariance matrix of group means. Each point represents one individual. Descending color intensity depicts the different time points (i.e. (12), 18, 24 and 36). Large points represent mean shape estimates for each cross type (A) or sex (B) at each trajectory point, and are connected in chronological order
Since the onset of sexual maturation occurred during the experiment and sex had an important impact on shape (Fig. 2, SM9), ontogenetic trajectories for both sexes were additionally explored. PTA showed significant differences in trajectory location, directionality and shape between sexes (p = 0.001, Pr > angle = 0.001 and Pr > d = 0.001 respectively), although absolute path distances did not differ (Pr > d = 0.94) (Fig. 5B). At month 18, mean shapes of both sexes laid practically on the same point in the morphospace. However, at month 24, they diverged along PC2, and differences increased markedly towards month 36, where the great morphological disparity seemed to be explained, to a larger degree, by sexual dimorphism. Additionally, morphological disparity in males was not only larger, but seemed to increase at a higher rate than morphological disparity in females (Table SM8, fig. SM8.1).
Discussion
In a long-running common garden experiment involving Arctic charr ecomorphs from lake Thingvallavatn (Iceland), we found that traits traditionally associated with benthic-limnetic adaptations have a genetic component. Interestingly, throughout ontogeny reciprocal hybrids between PL and SB showed intermediate, transgressive and parental-like phenotypes, indicating that the phenotypic outcome of hybrids is dynamic across time. Additionally, the onset of sexual maturation triggers differences in sex ontogenetic trajectories and shape variation at different time points. Taken together our results suggest that the interplay between traits associated with benthic-limnetic adaptations and traits associated with sexual dimorphism is complex and dynamic throughout ontogeny.
We asked whether differences in traits traditionally associated with benthic-limnetic adaptations were present in the different cross types. Geometric morphometric analyses revealed that traits associated to benthic-limnetic adaptations were detectable at each time point between SB and PL. These traits mostly comprise differences in the trophic apparatus, SB individuals having subterminal jaws, rounder snouts and deeper heads and bodies, whereas PLs were characterised for terminal jaws, pointed snouts and elongated heads and bodies. This result was in line with a recent study showing morphological differences in craniofacial morphology between PL and SB charr embryos reared in common garden conditions even before first feeding (Ponsioen, 2020). The genetic differences causing this phenotypic variation remain unknown and are likely to be complex. For instance, complex genetic architectures modulate distinct craniofacial structures in response to different selective pressures in benthic-limnetic cichlids from lake Malawi (Albertson & Kocher, 2005). Moreover, the functional differentiation between cichlids with suction vs. scrapping feeding apparatus is related to quantitative trait loci (QTL) ultimately influencing the shape of the articular and the dentary bones (Albertson et al., 2005; Parsons et al., 2011; Roberts et al., 2011). These QTLs, some having pleiotropic effect on the two bone structures, contain genes affecting bone development through different signalling pathways.
Patterns of shape variation in the reciprocal hybrids exhibited intermediate shapes at month 12, intermediate to transgressive shapes at month 18, and at month 24 most hybrids adopted a maternal phenotype. At month 36, major differences in shape variation did not occur between cross types, but between sexes. The different types of inheritance seen in the reciprocal hybrids indicates that the genetic component of the morphological traits unfolds in complex ways, and its expression, at least under the same environmental conditions, is asymmetrical and dependent on the parenthood. The hybrid’s use of either benthic or limnetic resources may be effective, but not constant across ontogeny. The ontogenetic niche shift (see MM, study system) between PL and SB charr is believed to occur in juveniles (Sandlund et al., 1992). This means that selection against hybrids with intermediate phenotypes may occur at month 12, but might be relaxed later in ontogeny, when hybrid phenotypic values tend towards the maternal morph. A recent study found that while the growth of hybrid embryos and early juveniles of PL and SB was similar to SB, covariance patterns between head shape and a set of traits including size was closer to PL’s (Horta-Lacueva et al., 2021). Our long-running common garden experiment further indicates that this complexity persists throughout ontogeny.
The onset of sexual maturation plays a key role in explaining shape variation across ontogeny, especially in later stages. At month 36, extensive differences in shape were found between males and females, pointing towards strong genetic basis to sexual dimorphism. Members of the Salmonidae family are characterised by a marked sexual dimorphism, which is thought to be, to some extent, due to sexual selection (Fleming, 1996; Gaudemar, 1998). Classic examples of pronounced sexual traits in salmonids can be mostly found in males. These are often larger body sizes, pointier or even hooked snouts, exaggerated humps and bright colorations, which are thought to have arisen as a result of high male densities and competition. At month 36 males showed similar phenotypes to the ones outlined above, namely pointier snouts, larger heads and humped dorsal area. Moreover, the increase in morphological disparity of the males relative to the females may indicate that the development of secondary sexual traits in males is more heterogenous and that the mechanisms leading to it are complex (see e.g., Woram et al., 2003; Sutherland et al., 2019).
It is worth noting that the dynamic patterns of sexual dimorphism and traits associated with benthic-limnetic adaptations are reversed (i.e., the relative load of cross type decreased while the relative load of sex increased) alluding that the developmental programs driving sexual maturation and the processes driving benthic-limnetic morphological divergence are decoupled. Such view is further supported by both ecomorph and sex ontogenetic trajectories: mean shapes of different morphs converge at month 36, as morphological disparity dramatically increases, and sex ontogenetic trajectories diverge. Further evidence for decoupling of sexual maturation and benthic-limnetic morphological divergence can be found in the non-significant interaction between sex and cross (SM3), indicating that the cross type and sex independently affect shape.
With our data, we were not able to determine whether sexual dimorphism in the Thingvallavatn system has originated prior-, post-ecological diversification, or a combination of both. Considering Arctic charr colonised different water bodies in Iceland and that it parallelly evolved distinct ecomorphs (Gíslason et al., 1999; Jacobs et al., 2020; Kapralova et al., 2011; Snorrason & Skúlason, 2004), one can argue that what we observe is the result of ancestral sexual dimorphism and subsequent adaptive radiation (for other systems, see: Aguirre et al., 2008; Lisle & Rowe, 1803). This is further supported by a widespread strong sexual dimorphism within the salmonid clade (Fleming, 1996; Gaudemar, 1998). Alternatively, secondary sexual traits evolving after sympatric radiations should be exclusive within each ecomorph, unless assortative mate choice occurs before trait divergence starts (Bolnick & Doebeli, 2003; Doorn & Weissing, 2002; Parsons et al., 2011; Slatkin, 1984). In our study, sexes mainly differed in relative head size, body depth and snout shape, but no differences were observed in the shape of the jaws, usually associated with benthic-limnetic feeding. Although the overlap between observed differences in males and females and between ecomorphs is minor, some trait variations such as relative head size and body depth appear to be common. Such an association may potentiate ecological adaptation within morphs, in line with other studies showing complex relationships between processes of speciation and sexual dimorphism (Cooper et al., 2011; Parsons et al., 2011). This complexity of different morphological traits across ontogeny points towards a combination of ancestral origin of sexual dimorphism followed by reinforcement of secondary sexual traits after sympatric divergence.
Conclusions
We found that phenotypic traits associated with benthic-limnetic adaptations in PL, SB and their hybrids are present and are genetically controlled at different time points. Sexual maturation is key in this scenario, since developmental programs driving the onset of the breeding season override adaptive traits, in similar environments at certain time points. The interplay between traits associated with ecological diversification and sexual maturation during development is complex and more efforts should be directed towards studying their relationship in this and other adaptive radiation systems, emphasising its dynamism throughout ontogeny.
Data availability
The data will be deposited onto the Dryad Digital Repository upon acceptance.
References
Ackermann, R. R., Rogers, J., & Cheverud, J. M. (2006). Identifying the morphological signatures of hybridization in primate and human evolution. Journal of Human Evolution, 51(6), 632–645.
Adams, C. E., & Huntingford, F. A. (2004). Incipient speciation driven by phenotypic plasticity? Evidence from sympatric populations of arctic charr. Biological Journal of the Linnean Society, 81(4), 611–618.
Adams, D. C., Rohlf, F. J., & Slice, D. E. (2013). A field comes of age: Geometric morphometrics in the 21st century. Hystrix, 24(1), 7–14.
Aguirre, W. E., Ellis, K. E., Kusenda, M., & Bell, M. A. (2008). Phenotypic variation and sexual dimorphism in anadromous threespine stickleback: Implications for postglacial adaptive radiation. Biological Journal of the Linnean Society, 95(3), 465–478.
Albertson, R. C., & Kocher, T. D. (2005). Genetic architecture sets limits on transgressive segregation in hybrid cichlid fishes. Evolution, 59(3), 686–690.
Albertson, R. C., Streelman, J. T., Kocher, T. D., & Yelick, P. C. (2005). Integration and evolution of the cichlid mandible: The molecular basis of alternate feeding strategies. Proceedings of the National Academy of Sciences, 102(45), 16287–16292.
Arnegard, M. E., McGee, M. D., Matthews, B., Marchinko, K. B., Conte, G. L., Kabir, S., et al. (2014). Genetics of ecological divergence during speciation. Nature, 511(7509), 307–311.
Baken, E. K., Collyer, M. L., Kaliontzopoulou, A., & Adams, D. C. (2021). geomorph v40 and gmShiny: enhanced analytics and a new graphical interface for a comprehensive morphometric experience. Methods in Ecology and Evolution. https://doi.org/10.1111/2041-210X.13723
Bell, M. A., & Travis, M. (2005). Hybridization, transgressive segregation, genetic covariation, and adaptive radiation. Trends in Ecology & Evolution, 20(7), 358–361.
Berner, D., Stutz, W. E., & Bolnick, D. I. (2010). Foraging trait (co) variances in stickleback evolve deterministically and do not predict trajectories of adaptive diversification. Evolution, 64(8), 2265–2277.
Berra, T. M. (2001). Freshwater fish distribution. Academic Press.
Bjoeru B, Sandlund O. 1995 Differences in morphology and ecology within a stunted Arctic char population. Nordic Journal of Freshwater Research (Sweden). New York
Blake, R., Law, T., Chan, K., & Li, J. (2005). Comparison of the prolonged swimming performances of closely related, morphologically distinct three-spined sticklebacks Gasterosteus spp. Journal of Fish Biology, 67(3), 834–848.
Bolnick, D. I., & Doebeli, M. (2003). Sexual dimorphism and adaptive speciation: Two sides of the same ecological coin. Evolution, 57(11), 2433–2449.
Bookstein, F. L. (1989). Principal warps: Thin-plate splines and the decomposition of deformations. IEEE Transactions on Pattern Analysis and Machine Intelligence, 11(6), 567–585.
Brachmann, M. K., Parsons, K., Skúlason, S., & Ferguson, M. M. (2021). The interaction of resource use and gene flow on the phenotypic divergence of benthic and pelagic morphs of Icelandic arctic charr (Salvelinus alpinus). Ecology and Evolution, 11(12), 7315–7334.
Butler, M. A., Sawyer, S. A., & Losos, J. B. (2007). Sexual dimorphism and adaptive radiation in Anolis lizards. Nature, 447(7141), 202–205.
Collyer ML, Adams DC. RRPP: Linear Model Evaluation with Randomized Residuals in a Permutation Procedure, R package version 1.1.2.” https://cran.r-project.org/package=RRPP. . 2021.
Collyer, M. L., & Adams, D. C. (2018). RRPP: An R package for fitting linear models to high-dimensional data using residual randomization. Methods in Ecology and Evolution. https://doi.org/10.1111/2041-210X.13029
Collyer, M. L., Davis, M. A., & Adams, D. C. (2020). Making heads or tails of combined landmark configurations in geometric morphometric data. Evolutionary Biology, 47(3), 193–205.
Cooper, I. A., Gilman, R. T., & Boughman, J. W. (2011). Sexual dimorphism and speciation on two ecological coins: Patterns from nature and theoretical predictions. Evolution, 65(9), 2553–2571.
De Gaudemar, B. (1998). Sexual selection and breeding patterns: Insights from salmonids (Salmonidae). Acta Biotheoretica, 46(3), 235–251.
De Lisle, S. P., & Rowe, L. (1803). Independent evolution of the sexes promotes amphibian diversification. Proceedings of the Royal Society B: Biological Sciences, 2015(282), 20142213.
de Villemereuil, P., Gaggiotti, O. E., Mouterde, M., & Till-Bottraud, I. (2016). Common garden experiments in the genomic era: New perspectives and opportunities. Heredity, 116(3), 249–254.
Dobzhansky, T. H. (1936). Studies on hybrid sterility II localization of sterility factors in drosophila pseudoobscura hybrids. Genetics, 21(2), 113–135.
Elgvin, T. O., Trier, C. N., Tørresen, O. K., Hagen, I. J., Lien, S., Nederbragt, A. J., et al. (2017). The genomic mosaicism of hybrid speciation. Science Advances, 3(6), e1602996.
Fleming, I. A. (1996). Reproductive strategies of Atlantic salmon: Ecology and evolution. Reviews in Fish Biology and Fisheries, 6(4), 379–416.
Gillespie, R. (2004). Community assembly through adaptive radiation in Hawaiian spiders. Science, 303(5656), 356–359.
Gíslason, D., Ferguson, M. M., Skúlason, S., & Snorrason, S. S. (1999). Rapid and coupled phenotypic and genetic divergence in Icelandic Arctic char (Salvelinus alpinus). Canadian Journal of Fisheries and Aquatic Sciences, 56(12), 2229–2234.
Grant, P. R., & Grant, B. R. (2002). Adaptive radiation of darwin’s finches: Recent data help explain how this famous group of Galapagos birds evolved, although gaps in our understanding remain. American Scientist, 90(2), 130–139.
Guðbrandsson, J., Franzdóttir, S. R., Kristjánsson, B. K., Ahi, E. P., Maier, V. H., Kapralova, K. H., et al. (2018). Differential gene expression during early development in recently evolved and sympatric Arctic charr morphs. PeerJ, 6, e4345.
Horta-Lacueva, Q.J.-B., Snorrason, S. S., Morrissey, M. B., Leblanc, C.A.-L., & Kapralova, K. H. (2021). Multivariate analysis of morphology, behaviour, growth and developmental timing in hybrids brings new insights into the divergence of sympatric Arctic charr morphs. BMC Ecology and Evolution, 21(1), 1–15.
Hulsey, C., Roberts, R., Loh, Y. H., Rupp, M., & Streelman, J. (2013). Lake Malawi cichlid evolution along a benthic/limnetic axis. Ecology and Evolution, 3(7), 2262–2272.
Jacobs, A., Carruthers, M., Yurchenko, A., Gordeeva, N. V., Alekseyev, S. S., Hooker, O., et al. (2020). Parallelism in eco-morphology and gene expression despite variable evolutionary and genomic backgrounds in a Holarctic fish. PLoS Genetics, 16(4), e1008658.
Janhunen, M., Peuhkuri, N., & Piironen, J. (2009). Morphological variability among three geographically distinct arctic charr (Salvelinus alpinus L) populations reared in a common hatchery environment. Ecology of Freshwater Fish, 18(1), 106–116.
Jones, F. C., Grabherr, M. G., Chan, Y. F., Russell, P., Mauceli, E., Johnson, J., et al. (2012). The genomic basis of adaptive evolution in threespine sticklebacks. Nature, 484(7392), 55–61.
Jonsson, B., Skúlason, S., Snorrason, S., Sandlund, O., Malmquist, H., Jónasson, P., et al. (1988). Life history variation of polymorphic arctic charr (Salvelinus alpinus) in Thingvallavatn, Iceland. Canadian Journal of Fisheries and Aquatic Sciences, 45(9), 1537–1547.
Kapralova, K. H., Gudbrandsson, J., Reynisdottir, S., Santos, C. B., Baltanás, V. C., Maier, V. H., et al. (2013). Differentiation at the MHCIIα and Cath2 loci in sympatric Salvelinus alpinus resource morphs in Lake Thingvallavatn. PLoS ONE, 8(7), e69402.
Kapralova, K. H., Jónsson, Z. O., Palsson, A., Franzdóttir, S. R., le Deuff, S., Kristjánsson, B. K., et al. (2015). Bones in motion: Ontogeny of craniofacial development in sympatric arctic charr morphs. Developmental Dynamics, 244(9), 1168–1178.
Kapralova, K., Morrissey, M., Kristjánsson, B., Ólafsdóttir, G. Á., Snorrason, S., & Ferguson, M. (2011). Evolution of adaptive diversity and genetic connectivity in arctic charr (Salvelinus alpinus) in Iceland. Heredity, 106(3), 472–487.
Kitano, J., Mori, S., & Peichel, C. L. (2007). Sexual dimorphism in the external morphology of the threespine stickleback (Gasterosteus aculeatus). Copeia, 2007(2), 336–349.
Losos, J. B. (2010). Adaptive radiation, ecological opportunity, and evolutionary determinism. The American Naturalist, 175(6), 623–639.
Losos, J. B., & Schneider, C. J. (2009). Anolis lizards. Current Biology, 19(8), R316–R318.
Malmquist, H., Snorrason, S., Skulason, S., Jonsson, B., Sandlund, O., & Jonasson, P. (1992). Diet differentiation in polymorphic arctic charr in Thingvallavatn Iceland. Journal of Animal Ecology, 61, 21–35.
McGee, M. D., & Wainwright, P. C. (2013). Sexual dimorphism in the feeding mechanism of threespine stickleback. Journal of Experimental Biology, 216(5), 835–840.
Meyer, A. (1993). Phylogenetic relationships and evolutionary processes in East African cichlid fishes. Trends in Ecology & Evolution, 8(8), 279–284.
Parsons, K. J., Sheets, H., Skúlason, S., & Ferguson, M. (2011). Phenotypic plasticity, heterochrony and ontogenetic repatterning during juvenile development of divergent arctic charr (Salvelinus alpinus). Journal of Evolutionary Biology, 24(8), 1640–1652.
Parsons, K. J., Skúlason, S., & Ferguson, M. (2010). Morphological variation over ontogeny and environments in resource polymorphic arctic charr (Salvelinus alpinus). Evolution & Development, 12(3), 246–257.
Parsons, K. J., Wang, J., Anderson, G., & Albertson, R. C. (2015). Nested levels of adaptive divergence: the genetic basis of craniofacial divergence and ecological sexual dimorphism. G3: Genes Genomes Genetics, 5(8), 1613–1624.
Ponsioen, L. (2020). Reproductive barriers between sympatric morphs of Arctic charr (Salvelinus alpinus) in lake Thingvallavatn. Iceland.
Pounder, K. C., Mitchell, J. L., Thomson, J. S., Pottinger, T. G., & Sneddon, L. U. (2018). Physiological and behavioural evaluation of common anaesthesia practices in the rainbow trout. Applied Animal Behaviour Science, 199, 94–102.
Reimchen, T., & Nosil, P. (2004). Variable predation regimes predict the evolution of sexual dimorphism in a population of threespine stickleback. Evolution, 58(6), 1274–1281.
Roberts, R. B., Hu, Y., Albertson, R. C., & Kocher, T. D. (2011). Craniofacial divergence and ongoing adaptation via the hedgehog pathway. Proceedings of the National Academy of Sciences, 108(32), 13194–13199.
Rohlf FJ. tpsDig, version 2.26. See bio sunysb edu/morph/soft-dataacq html. 2016.
Sandlund, O. T., Gunnarsson, K., Jónasson, P. M., Jonsson, B., Lindem, T., Magnússon, K. P., Malmquist, H. J., Sigurjónsdóttir, H., Skúlason, S., & Snorrason, S. S. (1992). The arctic charr salvelinus alpinus in thingvallavatn. Oikos, 64, 305–351.
Schluter, D. (2000). The ecology of adaptive radiation: OUP Oxford.
Seehausen, O. (2004). Hybridization and adaptive radiation. Trends in Ecology & Evolution, 19(4), 198–207.
Seehausen, O., & Wagner, C. E. (2014). Speciation in freshwater fishes. Annual Review of Ecology, Evolution, and Systematics, 45, 621–651.
Skúlason, S., Noakes, D. L., & Snorrason, S. S. (1989). Ontogeny of trophic morphology in four sympatric morphs of arctic charr Salvelinus alpinus in Thingvallavatn, Iceland. Biological Journal of the Linnean Society, 38(3), 281–301.
Skúlason, S., Snorrason, S. S., Ota, D., & Noakes, D. L. (1993). Genetically based differences in foraging behaviour among sympatric morphs of arctic charr (Pisces: Salmonidae). Animal Behaviour, 45(6), 1179–1192.
Slatkin, M. (1984). Ecological causes of sexual dimorphism. Evolution. https://doi.org/10.1111/j.1558-5646.1984.tb00327.x
Smith, T. B., & Skúlason, S. (1996). Evolutionary significance of resource polymorphisms in fishes, amphibians, and birds. Annual Review of Ecology and Systematics, 27(1), 111–133.
Snorrason, S. S., & Skúlason, S. (2004). Adaptive speciation in northern freshwater fishes. Adaptive speciation.
Snorrason, S. S., Skúlason, S., Jonsson, B., Malmquist, H. J., Jónasson, P. M., Sandlund, O. T., et al. (1994). Trophic specialization in Arctic charr Salvelinus alpinus (Pisces; Salmonidae): Morphological divergence and ontogenetic niche shifts. Biological Journal of the Linnean Society, 52(1), 1–18.
Snorrason, S. S., Skúlason, S., Sandlund, O. T., Malmquist, H. J., Jonsson, B., & Jonasson, P. (1989). Shape polymorphism in arctic charr Salvelinus Alpinus. Physiology and Ecology Japan, 1, 393–404.
Sutherland, B. J., Prokkola, J. M., Audet, C., & Bernatchez, L. (2019). Sex-specific co-expression networks and sex-biased gene expression in the salmonid Brook Charr Salvelinus fontinalis. G3: Genes, Genomes, Genetics, 9(3), 955–968.
Valentin, A., Penin, X., Chanut, J. P., Sévigny, J. M., & Rohlf, F. (2008). Arching effect on fish body shape in geometric morphometric studies. Journal of Fish Biology, 73(3), 623–638.
van Doorn, G. S., & Weissing, F. J. (2002). Ecological versus sexual selection models of sympatric speciation: A synthesis. Selection, 2(1–2), 17–40.
Vučić, T., Sibinović, M., Vukov, T. D., Tomašević Kolarov, N., Cvijanović, M., & Ivanović, A. (2019). Testing the evolutionary constraints of metamorphosis: The ontogeny of head shape in Triturus newts. Evolution, 73(6), 1253–1264.
Woram, R. A., Gharbi, K., Sakamoto, T., Hoyheim, B., Holm, L.-E., Naish, K., et al. (2003). Comparative genome analysis of the primary sex-determining locus in salmonid fishes. Genome Research, 13(2), 272–280.
Acknowledgements
We thank Kári H. Árnason, Rakel Þorbjörnsdóttir, and Christian Beuvard for the maintenance of the experimental setup at the rearing facility at Verið, Sauðárkrókur (Hólar University College, Iceland).
Funding
This work was fully funded by the Icelandic Centre of Research, RANNÍS (Icelandic Research Fund grant no. 1535–1533039 and 1535–1533090).
Author information
Authors and Affiliations
Contributions
MC conceptualised the study, conducted the analysis and wrote the manuscript. LP and QH collected the data, phenotyped the specimens and critically revised the manuscript. KHK conceived the study, established the crossing design, reared the embryos, collected the data and contributed to the writing of the manuscript. All authors gave their final approval for publication and agree to be accountable for the work therein.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interests.
Ethical Approval
The rearing and the experimental work was conducted in the facilities of Hólar University Aquaculture Research Station, which has an operational license under the Icelandic Aquaculture law (Law No. 71/2018). This law includes clauses of best practices for animal care and experimental work. Decisions on the sample size and on the design of the common-garden experiment were made to ensure that additional studies could be conducted with data collected on the same specimens.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
de la Cámara, M., Ponsioen, L., Horta-Lacueva, Q.J.B. et al. The Dynamic Ontogenetic Shape Patterns of Adaptive Divergence and Sexual Dimorphism. Evol Biol 50, 170–180 (2023). https://doi.org/10.1007/s11692-022-09592-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11692-022-09592-y