Abstract
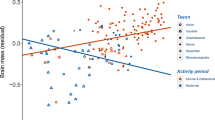
The evolution of brain size is constrained by the trade-off between the energetic costs allocated towards its maintenance and the cognitive advantages that come with a larger brain, leading to a paradox. The cognitive benefits of larger brains (e.g., high behavioural flexibility) mitigate extrinsic mortality factors, which may indirectly select for slower ageing that prolongs lifespan (“cognitive buffer hypothesis”). However, substantial energetic costs imposed by the maintenance of neural tissue is expected to compromise the energetic budget of large-brained organisms, and their investment in somatic maintenance and repair, thus accelerating ageing that shortens lifespan (the “disposable soma theory”). The relationship between lifespan and brain size has mostly been investigated in birds and mammals. Thus, whether these trade-offs express across ectothermic vertebrates remains to be addressed on a large-scale. Our study presents the first large-scale analysis of the brain size-lifespan relationship in ectothermic tetrapods (amphibians and reptiles). Using a dataset spanning 265 species, we performed phylogenetic linear models to investigate the predicted trade-off between variation in brain size and longevity. Our findings revealed a negative relationship between brain size and lifespan across reptiles, whereas no association was observed across amphibians. Thus, the relationship between life history and brain evolution in ectotherms does not follow the general pattern found across other vertebrates. Among ectotherms, the high metabolic cost of producing neural tissue seems to transcend the cognitive benefits of evolving a larger brain. Consequently, our findings suggest that natural selection favours optimization of the energetic economy over the fitness-advantages that cognitive benefits may offer.



Similar content being viewed by others
Data Accessibility Statement
All data used in the analyses and the associated metadata are available in Appendix S1. All codes used in the analyses are available in Appendix S3. Both data and codes in R software will be stored in Dryad and data DOI will be included at the end of the article.
Change history
17 March 2023
A Correction to this paper has been published: https://doi.org/10.1007/s11692-023-09599-z
References
Allman, J., McLaughlin, T., & Hakeem, A. (1993). Brain weight and lifespan in primate species. Proceedings of the National Academy of Sciences of the USA, 90, 118–122
Amiel, J. J., Tingley, R., & Shine, R. (2011). Smart moves: effects of relative brain size on establishment success of invasive amphibians and reptiles. PLoS One, 6(4), e18277
Barrickman, N. L., Bastian, M. L., Isler, K., & van Schaik, C. P. (2008). Life history costs and benefits of encephalization: a comparative test using data from long-term studies of primates in the wild. Journal of human evolution, 54, 568–590
Barton, R. A., & Capellini, I. (2011). Maternal investment, life histories, and the costs of brain growth in mammals. Proceedings of the National Academy of Sciences of the USA, 108, 6169–6174
Black, D. G. (1983). Encephalization of Australian lizards. Unpublished MSc thesis, Monash University, Clayton, Australia
Benson-Amram, S., Dantzer, B., Stricker, G., Swanson, E. M., & Holekamp, K. E. (2016). Brain size predicts problem-solving ability in mammalian carnivores. Proceedings of the National Academy of Sciences, 113(9), 2532–2537
Buechel, S. D., Boussard, A., Kotrschal, A., van der Bijl, W., & Kolm, N. (2018). Brain size affects performance in a reversal-learning test. Proceedings of the Royal Society B: Biological Sciences, 285(1871), 20172031
Burghardt, G. M., Chiszar, D., Murphy, J. B., Romano, J., Walsh, T., & Manrod, J. (2002). Behavioral complexity, behavioral development, and play. Komodo dragons: biology and conservation, 79–89
Carey, J. R. (2003). Longevity: the biology and demography of life span. Princeton University Press
Chapple, D. G. (2003). Ecology, life-history, and behavior in the Australian scincid genus Egernia, with comments on the evolution of complex sociality in lizards. Herpetological Monographs, 17(1), 145–180
De Meester, G., Huyghe, K., & Van Damme, R. (2019). Brain size, ecology and sociality: a reptilian perspective. Biological Journal of the Linnean Society, 126(3), 381–391
Deaner, R. O., Barton, R. A., & van Schaik, C. P. (2003). Primate brains and life histories renewing the connection (Pp. 233–265). In P. M. Kappeler And M. E. Pereira, eds. Primate life histories and socioecology. The University of Chicago Press, Chicago, IL
Deaner, R. O., Isler, K., Burkart, J., & Van Schaik, C. (2007). Overall brain size, and not encephalization quotient, best predicts cognitive ability across non-human primates. Brain, behavior and evolution, 70(2), 115–124
Dubois, E. (1913). On the relation between the quantity of brain and the size of the body in vertebrates. KNAB, 16, 647–668
Dunbar, R. I. M., & Shultz, S. (2007). Evolution in the social brain. Science, 317, 1344–1347
Eisenberg, J. F., & Wilson, D. E. (1981). Relative brain size and demographic strategies in didelphid marsupials. The American Naturalist, 118, 1–15
Ernst, C. H., Ernst, C. H., & Lovich, J. E. (2009). Turtles of the united states and Canada. JHU Press
Font, E., García-Roa, R., Pincheira-Donoso, D., & Carazo, P. (2019). Rethinking the effects of body size on the study of brain size evolution. Brain, behavior and evolution, 93(4), 182–195
Freckleton, R. P. (2002). On the misuse of residuals in ecology: regression of residuals vs. multiple regression. Journal of Animal Ecology, 542–545
Freckleton, R. P., Harvey, P. H., & Pagel, M. (2002). Phylogenetic analysis and comparative data: a test and review of evidence. The American Naturalist, 160, 712–726
Freckleton, R. P. (2009). The seven deadly sins of comparative analysis. Journal of Evolutionary Biology, 22(7), 1367–1375
García-Berthou, E. (2001). On the misuse of residuals in ecology: testing regression residuals vs. the analysis of covariance. Journal of Animal Ecology, 708–711
Gillooly, J. F., & McCoy, M. W. (2014). Brain size varies with temperature in vertebrates. PeerJ, 2, e301
González-Lagos, C., Sol, D., & Reader, S. M. (2010). Large‐brained mammals live longer. Journal of Evolutionary Biology, 23, 1064–1074
Gould, S. J. (1975). Allometry in primates, with emphasis on scaling and the evolution of the brain. Contributions to primatology, 5, 244–292
Green, A. J. (2001). Mass/length residuals: measures of body condition or generators of spurious results? Ecology, 82(5), 1473–1483
Güntürkün, O., Stacho, M., & Ströckens, F. (2020). The brains of reptiles and birds. Evolutionary Neuroscience, 159–212
Harraan, D. (1955). Aging: a theory based on free radical and radiation chemistry. Journal of Gerontology, 11, 298–300
Hartemink, N., & Caswell, H. (2018). Variance in animal longevity: contributions of heterogeneity and stochasticity. Population Ecology, 60, 89–99
Herculano-Houzel, S., Collins, C. E., Wong, P., & Kaas, J. H. (2007). Cellular scaling rules for primate brains. Proceedings of the National Academy of Sciences, 104(9), 3562–3567
Hofman, M. A. (1983). Energy metabolism, brain size and longevity in mammals. The Quarterly Review of Biology, 58, 495–512
Isler, K., & Van Schaik, C. P. (2006). Metabolic costs of brain size evolution. Biology letters, 2(4), 557–560
Isler, K., & van Schaik, C. P. (2009). The expensive brain: a framework for explaining evolutionary changes in brain size. Journal of Human Evolution, 57, 392–400
Jerison, H. J. (1973). Evolution of the brain and intelligence. New York: Academic Press
Jetz, W., & Pyron, R. A. (2018). The interplay of past diversification and evolutionary isolation with present imperilment across the amphibian tree of life. Nature Ecology & Evolution, 2, 850–858
Jiménez-Ortega, D., Kolm, N., Immler, S., Maklakov, A. A., & Gonzalez‐Voyer, A. (2020). Long life evolves in large brained bird lineages. Evolution. https://doi.org/10.1111/evo.14087
Jin, L., Zhao, L., Liu, W. C., Zeng, Y., & Liao, W. B. (2015). Evidence for the expensive-tissue hypothesis in the Omei Wood Frog (Rana omeimontis). The Herpetological Journal, 25(2), 127–130
Kotrschal, A., Corral-Lopez, A., & Kolm, N. (2019). Large brains, short life: selection on brain size impacts intrinsic lifespan. Biology Letters, 15, 20190137
Kotrschal, A., Rogell, B., Bundsen, A., Svensson, B., Zajitschek, S., Brännström, I. … Kolm, N. (2013a). Artificial selection on relative brain size in the guppy reveals costs and benefits of evolving a larger brain. Current Biology, 23(2), 168–171
Kotrschal, A., Rogell, B., Bundsen, A., Svensson, B., Zajitschek, S., Brännström, I. … Kolm, N. (2013b). The benefit of evolving a larger brain: big-brained guppies perform better in a cognitive task. Animal Behaviour, 86, e4
Kirkwood, T. B. L. (1992). The disposable soma theory: evidence and implications. Netherlands Journal of Zoology, 43, 359–363
Krilowicz, B. L., Glotzbach, S. F., & Heller, H. C. (1988). Neuronal activity during sleep and complete bouts of hibernation. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 255(6), R1008–R1019
Krilowicz, B. L., Edgar, D. M., & Heller, H. C. (1989). Action potential duration increases as body temperature decreases during hibernation. Brain research, 498(1), 73–80
Lázaro, J., Hertel, M., Sherwood, C. C., Muturi, M., & Dechmann, D. K. (2018). Profound seasonal changes in brain size and architecture in the common shrew. Brain Structure and Function, 223(6), 2823–2840
Leal, M., & Powell, B. J. (2012). Behavioural flexibility and problem-solving in a tropical lizard. Biology letters, 8(1), 28–30
Lefebvre, L., Whittle, P., Lascaris, E., & Finkelstein, A. (1997). Feeding innovations and forebrain size in birds. Animal Behaviour, 53, 549–560
Liao, W. B., Lou, S. L., Zeng, Y., & Kotrschal, A. (2016). Large brains, small guts: the expensive tissue hypothesis supported within anurans. The American Naturalist, 188, 693–700
Mai, C. L., & Liao, W. B. (2019). Brain size evolution in anurans: a review. Animal Biology, 69(3), 265–279
Magalhães, J. P. D., Costa, J., & Church, G. M. (2007). An analysis of the relationship between metabolism, developmental schedules, and longevity using phylogenetic independent contrasts. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 62, 149–160
Matějů, J., Kratochvíl, L., Pavelková, Z., Pavelková Řičánková, V., Vohralík, V., & Němec, P. (2016). Absolute, not relative brain size correlates with sociality in ground squirrels. Proceedings of the Royal Society B: Biological Sciences, 283(1827), 20152725
Meiri, S. (2018). Traits of lizards of the world: Variation around a successful evolutionary design. Global Ecology and Biogeography, 27, 1168–1172
Meiri, S. (2019). Endothermy, offspring size and evolution of parental provisioning in vertebrates. Biological Journal of the Linnean Society, 128, 1052–1056
Meiri, S., Avila, L., Bauer, A. M., Chapple, D. G., Das, I., Doan, T. M. … Morando, M. (2020). The global diversity and distribution of lizard clutch sizes. Global Ecology and Biogeography, 29(9), 1515–1530
Minias, P., & Podlaszczuk, P. (2017). Longevity is associated with relative brain size in birds. Ecology and Evolution, 7, 3558–3566
Mink, J. W., Blumenschine, R. J., & Adams, D. B. (1981). Ratio of central nervous system to body metabolism in vertebrates: its constancy and functional basis. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 241, R203–R212
Montgomery, S. H., Mundy, N. I., & Barton, R. A. (2016). Brain evolution and development: adaptation, allometry and constraint. Proceedings of the Royal Society B: Biological Sciences, 283(1838), 20160433
Nealen, P. M., & Ricklefs, R. E. (2001). Early diversification of the avian brain: body relationship. Journal of Zoology, 253, 391–404
Northcutt, R. G. (2013). Variation in reptilian brains and cognition. Brain, behavior and evolution, 82(1), 45–54
O’brien, R. M. (2007). A caution regarding rules of thumb for variance inflation factors. Quality & Quantity, 41, 673–690
O’connor, D., & Shine, R. (2003). Lizards in ‘nuclear families’: a novel reptilian social system in Egernia saxatilis (Scincidae). Molecular Ecology, 12(3), 743–752
Oliveira, B. F., São-Pedro, V. A., Santos-Barrera, G., Penone, C., & Costa, G. C. (2017). AmphiBIO, a global database for amphibian ecological traits. Scientific data, 4, 170123
Orme, D., Freckleton, R., Thomas, G., Petzoldt, T., Fritz, S., & Isaac, N. (2013). The caper package: comparative analysis of phylogenetics and evolution in R. R package version, 5, 1–36
Pincheira-Donoso, D., Harvey, L. P., Cotter, S. C., Stark, G., Meiri, S., & Hodgson, D. J. (2021). The global macroecology of brood size in amphibians reveals a predisposition of low‐fecundity species to extinction. Global Ecology and Biogeography, 30(6), 1299–1310
Platel, R. O. L. A. N. D. (1979). Brain weight-body weight relationships. Biology of the Reptilia, 9, 147–171
Reagan, D. P. (1992). Congeneric species distribution and abundance in a three-dimensional habitat: the rain forest anoles of Puerto Rico. Copeia, 2, 392–403
Ricklefs, R. E. (2010). Life-history connections to rates of aging in terrestrial vertebrates. Proceedings of the National Academy of Sciences, 107(22), 10314–10319
Rogell, B., Dowling, D. K., & Husby, A. (2020). Controlling for body size leads to inferential biases in the biological sciences. Evolution Letters, 4(1), 73–82
Sayol, F., Lefebvre, L., & Sol, D. (2016). Relative brain size and its relation with the associative pallium in birds. Brain, behavior and evolution, 87(2), 69–77
Scharf, I., Feldman, A., Novosolov, M., Pincheira-Donoso, D., Das, I., Böhm, M. … Meiri, S. (2015). Late bloomers and baby boomers: ecological drivers of longevity in squamates and the tuatara. Global Ecology and Biogeography, 24, 396–405
Slavens, F. L., … K. Slavens (1993). Reptiles and amphibians in captivity: breeding-longevity and inventory. Seattle: Slave ware
Sol, D., Székely, T., Liker, A., & Lefebvre, L. (2007). Big-brained birds survive better in nature. Proceedings of the Royal Society B, 274, 763–769
Sol, D., Bacher, S., Reader, S. M., & Lefebvre, L. (2008). Brain size predicts the success of mammal species introduced into novel environments. The American Naturalist, 172(S1), S63–S71
Sol, D. (2009). Revisiting the cognitive buffer hypothesis for the evolution of large brains. Biology Letters, 5(1), 130–133
Sol, D., Sayol, F., Ducatez, S., & Lefebvre, L. (2016). The life-history basis for behavioural innovations. Philosophical Transactions of the Royal Society B, 371, 20150187
Sonntag, M., & Arendt, T. (2019). Neuronal activity in the hibernating brain. Frontiers in Neuroanatomy, 13, 71
Stark, G., & Meiri, S. (2018). Cold and dark captivity: drivers of amphibian longevity. Global Ecology and Biogeography, 27, 1384–1397
Stark, G., Tamar, K., Itescu, Y., Feldman, A., & Meiri, S. (2018). Cold and isolated ectotherms: drivers of reptilian longevity. Biological Journal of the Linnean Society, 125, 730–740
Stark, G., Schwarz, R., & Meiri, S. (2020a). Does nocturnal activity prolong gecko longevity? Israel Journal of Ecology and Evolution, 1(aop), 1–8
Stark, G., Pincheira-Donoso, D., & Meiri, S. (2020b). No evidence for the ‘rate‐of‐living’ theory across the tetrapod tree of life. Global Ecology and Biogeography, 29, 857–884
Striedter, G. F. (2005). Principles of brain evolution. Sunderland, U.K: Sinauer Associates
Thireau, M. (1975). L’allométrie pondérale encéphalo-somatique chez les Urodèles. II. Relations interspécifiques
Trochet, A., Moulherat, S., Calvez, O., Stevens, V. M., Clobert, J., & Schmeller, D. S. (2014). A database of life-history traits of European amphibians. Biodiversity Data Journal, 2, e4123
Tsuboi, M., Husby, A., Kotrschal, A., Hayward, A., Buechel, S. D., Zidar, J. … Kolm, N. (2015). Comparative support for the expensive tissue hypothesis: big brains are correlated with smaller gut and greater parental investment in Lake Tanganyika cichlids. Evolution, 69, 190–200
Tsuboi, M., van der Bijl, W., Kopperud, B. T., Erritzøe, J., Voje, K. L., Kotrschal, A. … Kolm, N. (2018). Breakdown of brain–body allometry and the encephalization of birds and mammals. Nature Ecology & Evolution, 2, 1492–1500
Voituron, Y., et al. (2011). Extreme lifespan of the human fish (Proteus anguinus): A challenge for ageing mechanisms. Biology Letters, 7, 105–107
Wells, K. D. (2007). The ecology and behavior of amphibians. Illinois: University of Chicago Press
Wilkinson, G. S., & Adams, D. M. (2019). Recurrent evolution of extreme longevity in bats. Biology Letters, 15, 20180860
Wilkinson, A., & Huber, L. (2012). Cold-blooded cognition: reptilian cognitive abilities. The Oxford handbook of comparative evolutionary psychology (pp. 129–141). Oxford: Oxford University Press
Yu, X., Zhong, M. J., Li, D. Y., Jin, L., Liao, W. B., & Kotrschal, A. (2018). Large-brained frogs mature later and live longer. Evolution, 72, 1174–1183
Acknowledgements
We are grateful to Rachel Schwarz Stark and Simon Jamison for constructive discussion. We are also enormously grateful to Naomi Paz for English editing on an earlier draft of the manuscript.
Author information
Authors and Affiliations
Contributions
GS and DPD conceived the idea, outlined the hypotheses to be addressed and collected the data; GS led the preparation of the manuscript, devise the hypotheses, and performed most of the statistical analysis. DPD contributed at all stages of manuscript preparation.
Corresponding authors
Ethics declarations
Competing Interests statement
There are no competing interests among all authors for this manuscript.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Stark, G., Pincheira-Donoso, D. The Evolution of Brain Size in Ectothermic Tetrapods: Large Brain Mass Trades-Off with Lifespan in Reptiles. Evol Biol 49, 180–188 (2022). https://doi.org/10.1007/s11692-022-09562-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11692-022-09562-4