Abstract
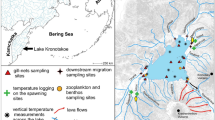
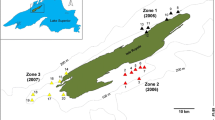
Within the sympatric evolution framework a range of ecological variables are considered as potential initiators and controllers of the diversification process. Here, we identify the proximate factors providing the reproductive isolation among seven sympatric ecomorphs of the genus Salvelinus charr dwelling in the Lake Kronotskoe basin (North-East Asia). We demonstrate that the slope profile of the lake tributaries determining the water flow velocity and position of the groundwater discharges serves as a barrier between the reproductive sites of the ecomorphs and provides the basis for selective pressure affecting the Lake Kronotskoe fish during spawning. The main characteristic under selection is a migratory ability, which is determined by the swimming performance and the amount of energy reserved in the body and depends on fish morphology and physiology. The flex points indicating abrupt slope changes along the spawning watercourse restrict the upstream migration of the groups having a comparatively low swimming performance and energy reserve. A thorough analysis of the ecomorphs’ migratory and spawning activity indicated two energy expenditure strategies: the fish could invest most of the energy either on migration or spawning. Our findings supported with data on fish ecology, morphology, and thyroid hormone status allow us to put forward a following hypothesis. We suggest that the interplay of spatially heterogeneous environmental variables affecting life history decisions via ecomorphological and physiological traits could serve as a trigger for the reproductive isolation among the ecomorphs in a single ecosystem.


Similar content being viewed by others
Data Availability
All the data obtained is included in this submission and it’s Supplement. Software, package and codes used in this work are freely available.
References
Adams, C. E., Fraser, D., Huntingford, F. A., Greer, R. B., Askew, C. M., & Walker, A. F. (1998). Trophic polymorphism amongst Arctic charr from Loch Rannoch Scotland. Journal of Fish Biology, 52(6), 1259–1271. https://doi.org/10.1111/j.1095-8649.1998.tb00970.x
Alekseyev, S. S., Gordeeva, N. V., Matveev, A. N., Samusenok, V. P., Vokin, A. I., & Yur’ev, A. L. (2014). Three sympatric forms of Arctic charr Salvelinus alpinus complex (Salmoniformes, Salmonidae) from Lake Kamkanda, Northern Transbaikalia. Journal of Ichthyology, 54(6), 384–408. https://doi.org/10.1134/S0032945214040018
Anteneh, W., Getahun, A., Dejen, E., Sibbing, F. A., Nagelkerke, L. A. J., De Graaf, M., et al. (2012). Spawning migrations of the endemic Labeobarbus (Cyprinidae, Teleostei) species of Lake Tana, Ethiopia: Status and threats. Journal of Fish Biology, 81(2), 750–765. https://doi.org/10.1111/j.1095-8649.2012.03362.x
Arakeliantz, A. D., & Tkachenko, O. V. (2012). Hydrological parameters of the Kronotskoye Lake (Kamchatka) at the beginning of the 21st century [in Russian]. Moscow University Bulletin. Series 5 Geography, 5, 77–83.
Armstrong, R. H. (1974). Migration of anadromous Dolly Varden (Salvelinus malma) in southeastern Alaska. Journal of the Fisheries Board of Canada, 31(4), 435–444.
Bain, M. B., & Stevenson, N. J. (1999). Aquatic habitat assessment. American Fisheries Society.
Baryshnikov, N. B. (2003). Hydraulic resistance in river channels and its relationship to channel processes. RGMU. [in Russian].
Baryshnikov, N. B. (2006). Hydraulic resistance in river channels and its relationship to channel processes. Prace Geograficzne, 116, 33–39.
Blake, R. W. (2004). Fish functional design and swimming performance. Journal of Fish Biology., 65(5), 1192–1222.
Blanton, M. L., & Specker, J. L. (2007). The Hypothalamic–pituitary–thyroid (HPT) axis in fish and its role in fish development and reproduction. Critical Reviews in Toxicology., 37, 97–115.
Bohlin, T., Pettersson, J., & Degerman, E. (2001). Population density of migratory and resident Brown trout (Salmo trutta) in relation to altitude: Evidence for a migration Cost. Journal of Animal Ecology, 70(1), 112–121.
Bolnick, D. I., & Fitzpatrick, B. M. (2007). Sympatric speciation: Models and empirical evidence. Annual Review of Ecology, Evolution, and Systematics, 38, 459–487.
Bond, M. H., Crane, P. A., Larson, W. A., & Quinn, T. P. (2014). Is isolation by adaptation driving genetic divergence among proximate Dolly Varden char populations? Ecology and Evolution, 4(12), 2515–2532. https://doi.org/10.1002/ece3.1113
Borazjani, I., & Sotiropoulos, F. (2009). On the role of form and kinematics on the hydrodynamics of self-propelled body/caudal fin swimming. Journal of Experimental Biology, 213, 89–107.
Boughman, J. W., & Svanbäck, R. (2017). Synergistic selection between ecological niche and mate preference primes diversification. Evolution, 71(1), 6–22. https://doi.org/10.1111/evo.13089
Braitseva, O. A., Melekestsev, I. V., Ponomareva, V. V., & Sulerzhitsky, L. D. (1995). Ages of calderas, large explosive craters and active volcanoes in the Kuril-Kamchatka region Russia. Bulletin of Volcanology, 57(6), 383–402. https://doi.org/10.1007/BF00300984
Brett, J. R. (1965). The swimming energetics of salmon. Scientific American, 213(2), 80–87.
Busarova, O. Y., Markevich, G. N., Knudsen, R., & Esin, E. V. (2017). Trophic differentiation of the nosed charr Salvelinus schmidti Viktorovsky, 1978 in Lake Kronotskoe (Kamchatka). Russian Journal of Marine Biology, 43(1), 57–64. https://doi.org/10.1134/S1063074017010023
Butlin, R. K., & Smadja, C. M. (2017). Coupling, reinforcement, and speciation. American Naturalist, 191(2), 155–172. https://doi.org/10.1086/695136
Campinho, M. A. (2019). Teleost metamorphosis: The role of thyroid hormone. Frontiers in Endocrinology (lausanne), 10, 383.
Carling, J., Williams, T. L., & Bowtell, G. (1998). Self-propelled anguilliform swimming: Simultaneous solution of the two-dimensional Navier-Stokes equations and Newton’s laws of motion. Journal of Experimental Biology, 201(23), 3143–3166.
Conover, W. J. (1999). Practical nonparameteric statistics (3rd ed., pp. 396–406). Wiley.
Coyne, J. A., & Orr, H. A. (2004). Speciation. Sinauer Associates.
Crossin, G. T., Hinch, S. G., Farrell, A. P., Higgs, D. A.,... & Healey, M. C. (2004). Energetics and morphology of sockeye salmon: Effects of upriver migratory distance and elevation. Journal of Fish Biology, 65(3), 788–810.
Dodson, J. J., Aubin-Horth, N., Thériault, V., & Páez, D. J. (2013). The evolutionary ecology of alternative migratory tactics in salmonid fishes. Biological Review, 88, 602–625.
Doenz, C. J., Krähenbühl, A. K., Walker, J., Seehausen, O., & Brodersen, J. (2019). Ecological opportunity shapes a large Arctic charr species radiation. Proceedings of the Royal Society b: Biological Sciences, 286(1913), 20191992. https://doi.org/10.1098/rspb.2019.1992
Dolgov, G. I. (1954). Determination of specific conductivity in practice of water research. VodGeo. [in Russian].
Dutil, J.-D. (1986). Energetic constraints and spawning interval in the anadromous Arctic charr (Salvelinus alpinus). Copeia, 4, 945–955.
Esin, E. V., Bocharova, E. S., Borisova, E. A., & Markevich, G. N. (2020). Interaction among morphological, trophic and genetic groups in the rapidly radiating Salvelinus fshes from Lake Kronotskoe. Evolutionary Ecology. https://doi.org/10.1007/s10682-020-10048-y
Esin, E. V., Markevich, G. N., & Pichugin, M. Y. (2018). Juvenile divergence in adaptive traits among seven sympatric fish eco-morphs arises before moving to different lacustrine habitats. Journal of Evolutionary Biology, 31(7), 1018–1034. https://doi.org/10.1111/jeb.13283
Galindo, D., Sweet, E., DeLeon, Z., Wagner, M., & DeLeon, A. (2019). Thyroid hormone modulation during zebrafish development recapitulates evolved diversity in danionin jaw protrusion mechanics. Evolution & Development, 21(5), 231–246.
Gordeev, I. I., Mikryakov, D. V., Silkina, N. I., Mikryakov, V. R., & Busarova, OYu. (2017). Content of immune complexes and the level of oxidative processes in blood and organs of chars in the Kronotskoe Lake (Kamchatka Peninsula). Arctic Environmental Research, 17(3), 204–211. https://doi.org/10.17238/issn2541-8416.2017.17.3.204
Hendry, A. P., & Berg, O. K. (1999). Secondary sexual characters, energy use, senescence, and the cost of reproduction in sockeye salmon. Canadian Journal of Zoology, 77(11), 1663–1675.
Herschy, R. W. (1995). Streamflow measurement. CRC Press.
Hill, T., & Lewicki, P. (2006). STATISTICS Methods and Applications. StatSoft.
Holzer, G., Besson, M., Lambert, A., Francois, L., Barth, P., Gillet, B., Hughes, S., Piganeau, G., Leullier, F., Viriot, L., Lecchini, D., & Laudet, V. (2017). Fish larval recruitment to reefs is a thyroid hormone-mediated metamorphosis sensitive to the pesticide chlorpyrifos. eLife Sciences. https://doi.org/10.7554/eLife.27595
Holzer, G., & Laudet, V. (2015). Thyroid hormones: A triple-edged sword for life history transitions. Current Biology, 25, R344–R347. https://doi.org/10.1016/j.cub.2015.02.026
Hughes, N. F. (2004). The wave-drag hypothesis: An explanation for size-based lateral segregation during the upstream migration of salmonids. Canadian Journal of Fisheries and Aquatic Sciences, 61(1), 103–109.
Iwata, M. (1995). Downstream migratory behavior of salmonids and its relationship with cortisol and thyroid hormones: A review. Aquaculture, 135(1–3), 131–139.
James, R. S., Little, A. G., Tallis, J., & Seebacher, F. (2016). Thyroid hormone influences muscle mechanics in carp (Cyprinus carpio) independently from SERCA activity. Journal of Experimental Biology, 219(Pt 18), 2806–2808. https://doi.org/10.1242/jeb.143529 Epub 2016 Jul 8 PMID: 27401758.
Jonsson, B., Skulason, S., Snorrason, S., Sandlund, O. T., Malmquist, H. J., Jonasson, P. M., et al. (1988). Life History Variation of Polymorphic Arctic Charr (Salvelinus alpinus) in Thingvallavatn, Iceland. Canadian Journal of Fisheries and Aquatic Sciences, 45(9), 1537–1547.
Kern, S., & Koumoutsakos, P. (2006). Simulations of optimized anguilliform swimming. Journal of Experimental Biology, 209, 4841–4857.
Klemetsen, A., Amundsen, P.-A., Knudsen, R., & Hermansen, B. (1997). A Profundal, Winter-Spawning Morph of Arctic charr Salvelinus alpinus (L.) in lake Fjellfrøsvatn, northern Norway. Nordic Journal of Freshwater Research, 73, 13–23.
Knudsen, R., Primicerio, R., Amundsen, P.-A., & Klemetsen, A. (2010). Temporal stability of individual feeding specialization may promote speciation. Journal of Animal Ecology, 79(1), 161–168. https://doi.org/10.1111/j.1365-2656.2009.01625.x
Kondratiuk, V. I. (1974). The climate at Kamchatka. HydroMetIsdat. [in Russian].
Leatherland, J. F., Down, N. E., Donaldson, E. M., & Dye, H. M. (1989). Changes in plasma thyroid hormone levels in Pink salmon, Oncorhynchus gorbuscha, during their spawning migration in the Fraser River (Canada). Journal of Fish Biology, 35, 199–205. https://doi.org/10.1111/j.1095-8649.1989.tb02969.x
Lema, S. C. (2020). Hormones, developmental plasticity, and adaptive evolution: Endocrine flexibility as a catalyst for ‘plasticity-first’ phenotypic divergence. Molecular and Cellular Endocrinology. https://doi.org/10.1016/j.mce.2019.110678
Lema, S. C., & Kitano, J. (2013). Hormones and phenotypic plasticity: Implications for the evolution of integrated adaptive phenotypes. Current Zoology, 59, 506–525.
Lennox, R. J., Eliason, E. J., Havn, T. B., Johansen, M. R.,... & Uglem, I. (2018). Bioenergetic consequences of warming rivers to adult Atlantic salmon Salmo salar during their spawning migration. Freshwater Biology, 63, 1381–1393.
Li, G., Müller, U. K., van Leeuwen, L., & Liu, H. (2012). Body dynamics and hydrodynamics of swimming fish larvae: A computational study. Journal of Experimental Biology, 215, 4015–4033.
Little, A. G., Kunisue, T., Kannan, K., & Seebacher, F. (2013). Thyroid hormone actions are temperature-specific and regulate thermal acclimation in zebrafish (Danio rerio). BMC Biology, 11, 26.
Manville, V., Hodgson, K. A., Houghton, B. F., Key, J. R. H., & White, J. D. L. (2000). Tephra, snow and water: Complex sedimentary responses at an active snow-capped stratovolcano, Ruapehu New Zealand. Bulletin of Volcanology, 62(4–5), 278–293. https://doi.org/10.1007/s004450000096
Markevich, G. N., Esin, E. V., & Anisimova, L. A. (2018). Basic description and some notes on the evolution of seven sympatric morphs of Dolly Varden Salvelinus malma from the Lake Kronotskoe Basin. Ecology and Evolution, 8(5), 2554–2567.
Markevich, G. N., Esin, E. V., Busarova, O. Y., Knudsen, R., & Anisimova, L. A. (2017). Diversity of nosed charrs Salvelinus malma (Salmonidae) of Lake Kronotskoe (Kamchatka). Journal of Ichthyology, 57(5), 675–687. https://doi.org/10.1134/S0032945217050101
Martín, J. S., Scheid, J. F., Takahashi, T., & Tucsnak, M. (2008). An initial and boundary value problem modeling of fish-like swimming. Archive for Rational Mechanics and Analysis, 188(3), 429–455. https://doi.org/10.1007/s00205-007-0092-2
Mesa, M. G., & Magie, C. D. (2006). Evaluation of energy expenditure in adult spring Chinook salmon migrating upstream in the Columbia River Basin: An assessment based on sequential proximate analysis. River Research and Applications, 22(10), 1085–1095.
Metsker, H. E. (1970). Fish versus culverts-some considerations for resource managers. Ogden.
Mochnacz, N. J., Schroeder, B. S., Sawatzky, C. D., & Reist, J. D. (2010). Assessment of northern Dolly Varden, Salvelinus malma malma (Walbaum, 1792), habitat in Canada. Canadian Manuscript Report of Fisheries and Aquatic Sciences, 2926, vi–48.
Müller, U. K., & van Leeuwen, J. L. (2004). Swimming of larval zebrafish: Ontogeny of body waves and implications for locomotory development. Journal of Experimental Biology, 207, 853–868.
Munakata, A. (2012). Migratory behaviors in masu salmon (Oncorhynchus masou) and the influence of endocrinological factors. Aqua-BioScience Monographs, 5, 29–65. https://doi.org/10.5047/absm.2012.00502.0029
Nosil, P., Harmon, L. J., & Seehausen, O. (2009). Ecological explanations for (incomplete) speciation. Trends in Ecology and Evolution, 24(3), 145–156. https://doi.org/10.1016/j.tree.2008.10.011
Pavlov, D. S., Pavlov, E. D., Ganzha, E. V., & Kostin, V. V. (2020). Change of rheoreaction and concentration of thyroid hormones in blood of juvenile Rainbow trout Oncorhynchus mykiss during starvation. Journal of Ichthyology, 60(2), 325–330.
Peake, S., McKinley, R. S., & Scruton, D. A. (1997). Swimming performance of various freshwater Newfoundland salmonids relative to habitat selection and fishway design. Journal of Fish Biology, 51, 710–723.
Pichugin, M. Y. (2015). Peculiarities of growth and skeletal system development of prelarvae, larvae, and fingerlings of Dolly Varden Trout Salvelinus malma malma inhabiting the rivers of Western Kamchatka in regard to the temperature regime of the spawning grounds. Journal of Ichthyology, 55(4), 549–566.
Pohlert, T. (2014). The Pairwise Multiple Comparison of Mean Ranks Package (PMCMR). R Package, 27(2019), 9.
Rolshausen, G., Segelbacher, G., Hobson, K. A., & Schaefer, H. M. (2009). Contemporary evolution of reproductive isolation and phenotypic divergence in sympatry along a migratory divide. Current Biology, 19(24), 2097–2101.
Rouleau, S., Glémet, H., & Magnan, P. (2010). Effects of morphology on swimming performance in wild and laboratory crosses of brook trout ecotypes. Functional Ecology, 24, 310–321.
Sandstrom, S. J., & Harwood, L. A. (2002). Studies of anadromous Dolly Varden (Salvelinus malma) (W.) of the Big Fish River, NT, Canada (pp. 1972–1994). Winnipeg, Manitoba.
Schluter, D. (2009). Evidence for ecological speciation and its alternative. Science, 32(5915), 737–741. https://doi.org/10.1126/science.1160006
Schmidt-Nielsen, K. (1972). Locomotion: Energy cost of swimming, flying, and running. Science, 177(4045), 222–228.
Seehausen, O. (2015). Process and pattern in cichlid radiations - inferences for understanding unusually high rates of evolutionary diversification. New Phytologist, 207(2), 304–312. https://doi.org/10.1111/nph.13450
Shkil, F. N., Dzerzhinskii, K. F., Abdissa, B., Borisov, V. B., & Smirnov, S. V. (2017). Notes on the breeding of large Lake Tana barbs (Labeobarbus spp.) in nature and laboratory. Ethiopian Journal of Biological Sciences, 16(1), 149–170.
Shkil, F. N., Lazebnyi, O. E., Kapitanova, D. V., Abdissa, B.,... & Smirnov, S. V. (2015). Ontogenetic mechanisms of explosive morphological divergence in the Lake Tana (Ethiopia) species flock of large African barbs (Labeobarbus; Cyprinidae; Teleostei). Journal of Developmental Biology, 46(5), 294–306.
Smith, J. M. (1966). Sympatric Speciation. The American Naturalist, 100(916), 637–650. https://doi.org/10.1086/282457
Sribnog, M. F. (1932). Resistance norms to velocity of natural watercourses and calculation of openings for large bridges. GosTransIzdat. [in Russian].
Taylor, E. B. (1999). Species pairs of north temperate freshwater fishes: Evolution, taxonomy, and conservation. Reviews in Fish Biology and Fisheries, 9(4), 299–324. https://doi.org/10.1023/A:1008955229420
Terhune, L. D. B. (1958). The mark VI groundwater standpipe for measuring seepage through salmon spawning gravel. Journal of the Fisheries Research Board of Canada, 15(5), 1027–1063. https://doi.org/10.1139/f58-056
Tytell, E. D., Hsu, C. Y., Williams, T. L., Cohen, A. H., & Fauci, L. J. (2010). Interactions between internal forces, body stiffness, and fluid environment in a neuromechanical model of lamprey swimming. Proceedings of the National Academy of Sciences of the United States of America, 107(46), 19832–19837. https://doi.org/10.1073/pnas.1011564107
Videler, J. J., & Weihs, D. (1982). Energetic advantages of burst-and-coast swimming of fish at high speeds. Journal of Experimental Biology, 97, 169–178.
Vlasov, G. M., & Chemikov, Y. F. (1950). Geomorphological zoning and the basic stages of landscapes formation during the Quaternary period in Kamchatkan range. Bulletins of Geographical Society, 82(3), 262–272.
Weihs, D. (1974). Energetic advantages of burst swimming of fish. Journal of Theoretical Biology, 1, 215–229.
Wickett, W. P. (1954). The Oxygen Supply to Salmon Eggs in Spawning Beds. Journal of the Fisheries Research Board of Canada, 11(6), 933–953. https://doi.org/10.1017/CBO9781107415324.004
Zadelenov, V. A., Shadrin, E. N., Matasov, V. V., & Romanov, V. I. (2014). On the biodiversity of chars from Big Norilsk lakes: Goggle-eyed char from the lake Sobach’e [in Russian]. In Current status of water bioresources (pp. 46–50).
Web Pages Citations
Geolkarta.ru N57-X, scale 1 : 200 000 tile. http://www.geolkarta.ru/list_200.php?idlist=N-57-X
Geolkarta.ru N57-XI, 1 : 200 000 tile. http://www.geolkarta.ru/list_200.php?idlist=N-57-XI
Geolkarta.ru N57-XVI, 1 : 200 000 tile. http://www.geolkarta.ru/list_200.php?idlist=N-57-XVI
Geolkarta.ru N57-XVII, 1 : 200 000 tile. http://www.geolkarta.ru/list_200.php?idlist=N-57-XVII
Acknowledgements
This work would have been impossible without the support of Kronotsky Reserve. Special thanks to: Alexey Serov, Fedor Kazanskiy, Dariya Panicheva, Evgeniy Rudnev, Vladimir Fedosov, Gleb Sedash, Andrey Bush, Rune Knudsen, Andrey Ivanov, Iliya Sharikov, and many others who helped us during the field and indoor investigations. The authors are grateful to Tatiana Gavrilova and Maria Dubkova for a careful reading of the manuscript.
Funding
Financial support was rendered by RSF Grant No.18-74-10085.
Author information
Authors and Affiliations
Contributions
G.N.M. and E.V.E designed the study, conducted the field work, and wrote the major part of the manuscript. D.V.Z. processed the ecological modeling and statistical analysis, and wrote the part of manuscript. F.N.S. measured the hormone content and worked with the manuscript design. U.K.S. worked on the data analysis, wrote the part of the manuscript. L.A. did the part of field investigations A.A.S. did the part of hydrological measurements, processed that data and worked on the manuscript draft. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
The authors declare that the materials used were obtained complying with the current laws of Russian Federation. The charr Salvelinus malma is not an endangered or protected species. Following the Federal law “On Fisheries and Conservation of Aquatic Biological Resources” №166-ФЗ, the non-commercial fishing of this charr does not require any permissions. All field procedures with fish were approved by the ethics committee of the Severtsov Institute of Ecology and Evolution, Russian Academy of Sciences.
Informed Consent
All authors agree to participate in the manuscript and obtain consent from the responsible authorities at the institute where the work has been carried out.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendix
Appendix
Satellite Data Processing
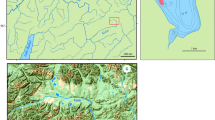
The above sea level (a.s.l.) marks were obtained from the global digital elevation model (Global DEM). These data were granted by TanDEM-X Science Service System (12 m (0.4 arcsec) tiles N54E159, N54E160 and N55E160 hydrology DEM) and processed in ArcMap v.10.1 (ESRI). The relative elevation marks were measured with an accuracy of ± 0.5 m, and ten measurements were processed for each point at each locality and averaged. Such marks were acquired for the (i) upper, middle, and lower course of spawning site; (ii) for contiguous 1 km sections starting from the downstream margin of the spawning site to the river mouth; and (iii) for 100–200 m sections from the confluence to 1 km upstream for each tributary. Elevation marks were verified with the elevation benchmarks from the 1:100.000 topographic maps. Finally, these data were combined to estimate the slopes along morph’s spawning migration path. Stream velocity was calculated for each section (see below) and verified with the field measurements. River section length and width were obtained from precise satellite images in SASPlanet (GoogleEarth, BingMaps and HereMaps data combined). The width was measured every 100 m for each section and averaged for the 1 km sections.
Calculation of the Riverbed Slope and Water Flow Velocity
The slope of each section i (Ii) was calculated as:
where Li is the length of the section varied between 100 m in proximity of the branching points and 1 km in other parts, and ΔHi – the difference in the absolute altitude marks. The average stream velocity in each section (Vi) was calculated as:
where kChezy is the Chezy-coefficient relating the slope (I) and some effective average depth (h) with the average water flow velocity at the given i-th river section (Baryshnikov, 2003). The Chezy-coefficient was calculated as follows:
where kroug is the roughness coefficient reflecting the friction of flowing water on the river bed composed of the different-sized floor grounds. The values of the roughness coefficient are empirically measured and tabulated (Baryshnikov, 2006; Sribnog, 1932).
The average depth of each section (hi) is tough to measure experimentally, not only due to the large amount of work but also due to the “effective” nature of this parameter describing the mean properties of a rather long river section. Therefore, we have assessed the average depth of each i-th river section as follows (Baryshnikov, 2003):
where Qi is the average water discharge, and Bi – the average river width. The latter was directly measured using the satellite images, while the former was assessed, assuming the discharges to be approximately constant at the sections lacking the tributaries and sum up at the confluence of two watercourses. The discharges were estimated at the control sections near each confluent point:
basing on the directly measured averaged parameters: actual average river depth (hi), actual mean river width (Bi), and average velocity of the water flow (Vi).
The values of the slopes and integral velocity obtained for 16 spawning sites were statistically tested by H-test and post-hoc Van der Waerden test for multiple comparisons of small-numbered samples in PMCMR module of R (Pohlert, 2014).
Energy Cost of Spawning Migration and Reproduction
The Stokes friction force for the “spherical fish” traveling through the water thick could be written as:
where Ffric is the friction force, η—the dynamic viscosity of the water, R – the sphere radius, and V—stream velocity. We have assumed the “radius” of the fish to be one half of the maximal fish section diameter. The total work of fish against the friction force could be divided into two terms: the work during active migration (Eburst) and the work during resting periods (Ecoast). Consequently, Eburst and Ecoast could be calculated separately. Denoting an average length of a single burst with respect to the ground as Lb and the burst speed as Vb, then the real length (Lw,i) of the i-th burst in respect to the water would be:
where Vw,i is the velocity of the water flow. Thus, the total work (Aburst,i) against the friction forces during the i-th burst can be derived as:
Thus, the total energy spent for active upstream migration would be:
For salmonids, the average burst speed (Vb) equals several meters per second (Brett, 1965; Metsker, 1970). This parameter depends significantly on the fish size and its overall activity; thereby it seems appropriate to measure it separately for each morph. Moreover, the burst speed also depends on the fish’s physiological condition and changes during the spawning migration. Thus, measurement of this parameter is a very complicated experimental task. To overcome this problem, we calculated Aburst for the unbelievable low Vburst of 1 m/s and unbelievable high Vburst of 10 m/s. We used the obtained values as lower- and upper-bound estimates of Aburst. However, getting ahead, we can state that absolute values of Aburst appear too small relatively to other terms, and assumed inaccuracies did not affect the overall conclusions.
The work against friction forces during coasting periods (Acoast,i) can be calculated in a similar way. Let us assume that all resting periods have approximately the same duration (tcoast). As we assume that the fish does not migrate during resting periods, the distance with respect to the water (Lcoast,i) during the i-th rest period would be just:
as the fish do not move downstream on average. The corresponding work against the friction forces (Acoast,i) would be:
The total sum of all Acoast,i leads to the lower-bound estimation of the corresponding mean energy expenditure:
where N is the number of burst and rest periods.
The product of the average length of the burst (Lb) and the number of such bursts (N) equals the river length (LR). Consequently, the increase of Lb correspondingly decreases N. Exactly the same, the product of the average time of the coast (tcoast) and the number (N) of the coast periods (and also bursts) is the spawning migration time (tm), which is also known from the natural observations. Thus, tcoast increase correspondingly diminishes N and causes the same increase of the burst length (Lb), as the number of bursts and coast periods (N) equals each other:
The exact values of tcoast and Lb are tough to define experimentally. Depending on the riverbed structure and the pattern of the velocity field distribution in it, these parameters can vary in a wide range. However, in our terminally simplified approach, the estimate on the Ecoast and Eburst would depend on the values of tcoast and Lb, if only Lb would be comparable with the spatial resolution of the data on the water flow speed along the river. Indeed, if Lb is smaller than the discretization scale, the twofold (for example) decrease of Lb would cause a twofold increase in the number of the same terms multiplied on the twofold smaller value in the expression for Eburst. So, the result would not change. In our case, the best spatial resolution of the slope estimating was 100–200 m in tributaries' proximity, which is far longer than could be even proposed for a single burst. Therefore, the accuracy of the slope and water flow velocity estimation restricts the accuracy of the proposed approach and makes determining the exact values of tcoast and Lb senseless. So, we have assumed Lb to be equal to 10 m and calculated the corresponding tcoast for each migration route and time.
Besides the migration's energy expenditure, fish have to resist the water flow during the spawning itself, also requiring some energy to be spent (Espawn). The expression for Espawn could be derived similarly to the Ecoast, but the time period (tcoast), in this case would be equal to the total spawning duration (tspawn):
Finally, the total spawning energy cost (Etotal) could be assessed as:
The total estimated spawning energy costs for different morphs (totally 16 calculations) were statistically compared using Van der Waerden test in PMCMR module of R.
Rights and permissions
About this article
Cite this article
Markevich, G.N., Zlenko, D.V., Shkil, F.N. et al. Natural Barriers and Internal Sources for the Reproductive Isolation in Sympatric Salmonids from the Lake–River System. Evol Biol 48, 407–421 (2021). https://doi.org/10.1007/s11692-021-09546-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11692-021-09546-w