Abstract
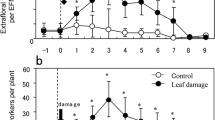
In addition to direct defenses, some plant species provide extrafloral nectar (EF-nectar) and/or food bodies (lipid-rich particles) to attract ants for their own indirect defenses. To ascertain why such plants use indirect defenses, we investigated the respective costs of direct and indirect defenses of Mallotus japonicus seedlings grown with and without ants present. Mallotus japonicus plants growing with ants present (ant-present) secreted larger volumes of EF-nectar, containing greater amounts of sugars, as an indirect defense trait. These plants also showed chemical defensive traits, such as the number of pellucid dots and the amount of accumulated phenolics, to a lesser degree than plants without ants (ant-absent) did. Moreover, the ant-present plants grew faster. The estimated amounts of EF-nectar sugars and food bodies were small compared to the amount of phenolics. Plant biomass was correlated negatively with pellucid dot density and phenolic concentration. Plant height was correlated negatively with phenolic concentration. Moreover, leaf biomass was correlated negatively with trichome density. Taken together, these results suggest a tradeoff between the expression of direct defense traits and plant growth. Mallotus japonicus achieves more rapid growth with ants present. We propose that this occurs because these ants provide low-cost indirect defenses allowing plants to re-allocate their energy from direct defenses to growth instead. This mutual benefit apparently facilitates ant–plant defensive mutualism.
Similar content being viewed by others
References
Agrawal, A. A. (2011). Current trends in the evolutionary ecology of plant defence. Functional Ecology, 25(2), 420–432.
Barton, A. M. (1986). Spatial variation in the effect of ants on extrafloral nectary plant. Ecology, 67(2), 495–504.
Bentley, B. L. (1977). Extrafloral nectaries and protection by pugnacious bodyguards. Annual Reviews Ecology, Evolution and Systematics, 8, 407–427.
Betancur, L., Singh, B., Rapp, R. A., Wendel, J. F., Marks, M. D., Roberts, A. W., & Haigler, C. H. (2010). Phylogenetically distinct cellulose synthase genes support secondary wall thickening in Arabidopsis shoot trichomes and cotton fiber. Journal of Integrative Plant Biology, 52(2), 205–220.
Bixenmann, R. J., Coley, P. D., & Kursar, T. A. (2011). Is extrafloral nectar production induced by herbivores or ants in a tropical facultative ant–plant mutualism? Oecologia, 165(2), 417–425.
Braam, J. (2005). In touch: plant responses to mechanical stimuli. New Phytologist, 165(2), 373–389.
Bronstein, J. L., & Barbosa, P. (2002). Multitrophic/multispecies mutualistic interactions: the role of non-mutualists in shaping and mediating mutualisms. In T. Tscharntke & B. A. Hawkins (Eds.), Multitrophic level interactions (pp. 44–66). New York: Cambridge University Press.
Chanam, J., Sheshshayee, M. S., Kasinathan, S., Jagdeesh, A., Joshi, K. A., & Borges, R. M. (2014). Nutritional benefits from domatia inhabitants in an ant–plant interaction: interlopers do pay the rent. Functional Ecology, 28(5), 1107–1116.
Chehab, E. W., Yao, C., Henderson, Z., Kim, S., & Braam, J. (2012). Arabidopsis touch-induced morphogenesis is jasmonate mediated and protects against pests. Current Biology, 22(8), 701–706.
Darwin, C. (1862). On the various contrivances by which British and foreign orchids are fertilised by insects, and on the good effects of intercrossing. London: John Murray.
Degnan, P. H., Yu, Y., Sisneros, N., Wing, R. A., & Moran, N. A. (2009). Hamiltonella defensa, genome evolution of protective bacterial endosymbiont from pathogenic ancestors. Proceedings of the National Academy of Sciences of the United States of America, 106(22), 9063–9068.
Development, R. (2012). R: a language and environment for statistical computing. Vienna: Austria.
Doebeli, M., & Knowlton, N. (1998). The evolution of interspecific mutualisms. Proceedings of the National Academy of Sciences of the United States of America, 95(15), 8676–8680.
Dudt, J. F., & Shure, D. J. (1994). The influence of light and nutrients on foliar phenolics and insect herbivory. Ecology, 75(1), 86–98.
Dyer, L. A., Dodson, C. D., Beihoffer, J., & Letourneau, D. K. (2001). Trade-offs in antiherbivore defenses in Piper cenocladum: ant mutualists versus plant secondary metabolites. Journal of Chemical Ecology, 27(3), 581–592.
Federle, W., Riehle, M., Curtis, A. S., & Full, R. J. (2002). An integrative study of insect adhesion: mechanics and wet adhesion of pretarsal pads in ants. Integrative and Comparative Biology, 42(6), 1100–1106.
Feeny, P. (1970). Seasonal changes in oak leaf tannins and nutrients as a cause of spring feeding by winter moth caterpillars. Ecology, 51(4), 565–581.
Feng, Y. L., Lei, Y. B., Wang, R. F., Callaway, R. M., Valiente-Banuet, A., Inderjit, Li, Y. P., et al. (2009). Evolutionary tradeoffs for nitrogen allocation to photosynthesis versus cell walls in an invasive plant. Proceedings of the National Academy of Sciences of the United States of America, 106(6), 1853–1856.
Guhling, O., Kinzler, C., Dreyer, M., Bringmann, G., & Jetter, R. (2005). Surface composition of myrmecophilic plants: cuticular wax and glandular trichomes on leaves of Macaranga tanarius. Journal of Chemical Ecology, 31(10), 2323–2341.
Heil, M. (2013). Let the best one stay: screening of ant defenders by Acacia host plants functions independently of partner choice or host sanctions. Journal of Ecology, 101(3), 684–688.
Heil, M., Fiala, B., Baumann, B., & Linsenmair, K. E. (2000). Temporal, spatial and biotic variations in extrafloral nectar secretion by Macaranga tanarius. Functional Ecology, 14(6), 749–757.
Heil, M., Fiala, B., Kaiser, W., & Linsenmair, K. E. (1998). Chemical contents of Macaranga food bodies adaptations to their role in ant attraction and nutrition. Functional Ecology, 12(1), 117–122.
Heil, M., Fiala, B., Linsenmair, K. E., Zotz, G., Menke, P., & Maschwitz, U. (1997). Food body production in Macaranga triloba (Euphorbiaceae): a plant investment in anti-herbivore defence via symbiotic ant partners. Journal of Ecology, 85(6), 847–861.
Heil, M., González-Teuber, M., Clement, L. W., Kautz, S., Verhaagh, M., & Bueno, J. C. S. (2009). Divergent investment strategies of Acacia myrmecophytes and the coexistence of mutualists and exploiters. Proceedings of the National Academy of Sciences of the United States of America, 106(43), 18091–18096.
Heil, M., Greiner, S., Meimberg, H., Krüger, R., Noyer, J. L., Heubl, G., et al. (2004). Evolutionary change from induced to constitutive expression of an indirect plant resistance. Nature, 430(6996), 205–208.
Heil, M., & Karban, R. (2009). Explaining evolution of plant communication by airborne signals. Trends in Ecology & Evolution, 25(3), 134–144.
Heil, M., Koch, T., Hilpert, A., Fiala, B., Boland, W., & Linsenmair, K. E. (2001). Extrafloral nectar production of the ant-associated plant, Macaranga tanarius, is an induced, indirect defensive response elicited by jasmonic acid. Proceedings of the National Academy of Sciences of the United States of America, 98(3), 1083–1088.
Hilker, M., & Meiners, T. (2010). How do plants “notice” attack by herbivorous arthropods? Biological Reviews, 85, 267–280.
Hölldoble, B., & Palmer, J. M. (1989). Footprint glands in Amblyopone australis (Formicidae, Ponerinae). Psyche, 96(1–2), 111–122.
Izaguirre, M. M., Mazza, C. A., Astigueta, M. S., Ciarla, A. M., & Ballaré, C. L. (2013). No time for candy: passionfruit (Passiflora edulis) plants down-regulate damage-induced extra floral nectar production in response to light signals of competition. Oecologia, 173(1), 213–221.
Julkunen-Tiitto, R. (1985). Phenolic constituents in the leaves of northern willows: methods for the analysis of certain phenolics. Journal of Agricultural and Food Chemistry, 33(2), 213–217.
Katayama, N., & Suzuki, N. (2011). Anti-herbivory defense of two Vicia species with and without extrafloral nectaries. Plant Ecology, 212(5), 743–752.
Koptur, S. (1985). Alternative defenses against herbivores in Inga (Fabaceae: Mimosoideae) over an elevational gradient. Ecology, 66(5), 1639–1650.
Koptur, S. (1992). Extrafloral nectary-mediated interactions between insects and plants. In E. A. Bernays (Ed.), Insect–plant interactions (Vol. 4, pp. 81–129). Boca Raton: CRC Press.
Leigh, E. G, Jr. (2010). The evolution of mutualism. Journal of Evolutionary Biology, 23(12), 2507–2528.
Lim, T. Y., Lim, Y. Y., & Yule, C. M. (2009). Evaluation of antioxidant, antibacterial and anti-tyrosinase activities of four Macaranga species. Food Chemistry, 114(2), 594–599.
Martín-Closas, L., Toro, F. J., Calvó, G., & Pelacho, A. M. (2003). Effect of methyl jasmonate on the first developmental stages of globe artichoke. Acta Horticulturae, 660, 185–190.
Millán-Cañongo, C., Orona-Tamayo, D., & Heil, M. (2014). Phloem sugar flux and jasmonic acid-responsive cell wall invertase control extrafloral nectar secretion in Ricinus communis. Journal of Chemical Ecology, 40(7), 760–769.
O’Dowd, D. J. (1979). Foliar nectar production and ant activity on a neotropical tree, Ochroma pyramidale. Oecologia, 43(2), 233–248.
O’Dowd, D. J. (1982). Pearl bodies as ant food: an ecological role for some leaf emergences of tropical plants. Biotropica, 14(1), 40–49.
Palmer, T. M., Stanton, M. L., Young, P. T., Goheen, J. R., Pringle, R. M., & Karban, R. (2008). Breakdown of an ant-plant mutualism follows the loss of large herbivores from an African savanna. Science, 319(5860), 192–195.
Radhika, V., Kost, C., Mithöfer, A., & Boland, W. (2010). Regulation of extrafloral nectar secretion by jasmonates in lima bean is light dependent. Proceedings of the National Academy of Sciences of the United States of America, 107(40), 17228–17233.
Redman, A. M., Cipollini, D. F, Jr, & Schultz, J. C. (2001). Fitness costs of jasmonic acid-induced defense in tomato, Lycopersicon esculentum. Oecologia, 126(3), 380–385.
Rios, R. S., Marquis, R. J., & Flunker, J. C. (2008). Population variation in plant traits associated with ant attraction and herbivory in Chamaecrista fasciculata (Fabaceae). Oecologia, 156(3), 577–588.
Risch, S. J., & Rickson, F. (1981). Mutualism in which ants must be present before plants produce food bodies. Nature, 291(14), 149–150.
Rudgers, J. A., & Strauss, S. Y. (2004). A selection mosaic in the facultative mutualism between ants and wild cotton. Proceedings of the Royal Society B, 271(1556), 2481–2488.
Rutter, M. T., & Rausher, M. D. (2004). Natural selection on extrafloral nectar production in Chamaecrista fasciculata: the costs and benefits of a mutualism trait. Evolution, 58(12), 2657–2668.
Schilmiller, A. L., Last, R. L., & Pichersky, E. (2008). Harnessing plant trichome biochemistry for the production of useful compounds. Plant Journal, 54(4), 702–711.
Schupp, E. W., & Feener, D. H. (1991). Phylogeny, lifeform, and habitat dependence of ant-defended plants in a Panamanian forest. In C. R. Huxley & D. F. Cutler (Eds.), Ant–plant interactions (pp. 175–197). Oxford: Oxford Univ. Press.
Scott, P. (2008). Physiology and behaviour of plants. Chichester: Wiley.
Sirikantaramas, S., Yamazaki, M., & Saito, K. (2008). Mechanisms of resistance to self-produced toxic secondary metabolites in plants. Phytochemistry Reviews, 7(3), 467–477.
Stadler, B., & Dixon, T. (2008). Mutualism: ants and their insect partners. Cambridge: Cambridge University Press.
Strauss, S. Y., Rudgers, J. A., Lau, J. A., & Irwin, R. E. (2002). Direct and ecological costs of resistance to herbivory. Trends in Ecology & Evolution, 17(6), 278–285.
Toro, F. J., Martín-Closas, L., & Pelacho, A. M. (2003). Jasmonates promote cabbage (Brassica oleracea L. var Capitata L.) root and shoot development. Developments in Plant and Soil Sciences, 101, 77–83.
Vickery, M. L., & Vickery, B. (1981). Secondary plant metabolism. London: Macmillan.
Wagner, D., & Nicklen, E. F. (2010). Ant nest location, soil nutrients and nutrient uptake by ant-associated plants: does extrafloral nectar attract ant nests and thereby enhance plant nutrition? Journal of Ecology, 98(3), 614–624.
Washitani, I., & Takenaka, A. (1987). Gap-detecting mechanism in the seed germination of Mallotus japonicus (Thunb.) Muell. Arg., a common pioneer tree of secondary succession in temperate Japan. Ecological Research, 2(3), 191–201.
Wasternack, C. (2007). Jasmonates: an update on biosynthesis, signal transduction and action in plant stress response, growth and development. Annals of Botany, 100(4), 681–697.
Wittstock, U., & Gershenzon, J. (2002). Constitutive plant toxins and their role in defense against herbivores and pathogens. Current Opinion in Plant Biology, 5(4), 300–307.
Yamawo, A., Katayama, N., Suzuki, N., & Hada, Y. (2012a). Plasticity in the expression of direct and indirect defence traits of young plants of Mallotus japonicus in relation to soil nutritional conditions. Plant Ecology, 213(1), 127–132.
Yamawo, A., Suzuki, N., Tagawa, J., & Hada, Y. (2012b). Leaf ageing promotes the shift in defence tactics in Mallotus japonicus from direct to indirect defence. Journal of Ecology, 100(3), 802–809.
Yamawo, A., Tagawa, J., Hada, Y., & Suzuki, N. (2014). Different combinations of multiple defence traits in an extrafloral nectary-bearing plant growing under various habitat conditions. Journal of Ecology, 102(1), 238–247.
Acknowledgments
This work was supported in part by Research Fellowships from the Japan Society for the Promotion of Science for Young Scientists (234305) and (251712).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yamawo, A., Tokuda, M., Katayama, N. et al. Ant-Attendance in Extrafloral Nectar-Bearing Plants Promotes Growth and Decreases the Expression of Traits Related to Direct Defenses. Evol Biol 42, 191–198 (2015). https://doi.org/10.1007/s11692-015-9310-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11692-015-9310-2