Abstract
Purpose
The present paper describes two new genera and species of the parasitic copepod family Chondracanthidae Milne Edwards, 1840 based on specimens collected from two species of deep-sea fishes at a depth of 212 m off Suruga Bay, Japan. Avatar nishidai gen. et sp. nov. is described from the host fish Chaunax abei Le Danois, 1978 (Chaunacidae). Kokeshioides surugaensis gen. et sp. nov. is described from the host fish Setarches longimanus (Alcock, 1894) (Setarchidae).
Methods
Fresh specimens of chondracanthids were collected from the buccal cavity of two species of deep-sea fishes (fish hosts were frozen), Chaunax abei Le Danois, 1978 (Lophiiformes: Chaunacidae) and Setarches longimanus (Alcock, 1894) (Perciformes: Setarchidae), caught at a depth of 212 m in Suruga Bay, Japan (34° 37′48.87″ N, 138° 43′2.958″ E). Both the species are described and illustrated based on ovigerous females.
Results
The genus Avatar gen. nov. can readily be distinguished from all other chondracanthid genera by the following combination of features: cephalothorax slightly wider than long with anterior pair of large and posterior pair of small lateral lobes, and two pairs of ventro-lateral processes; the very posteriormost part of the first pedigerous somite contributes to the neck; cylindrical trunk with two pairs of blunt proximal fusiform processes; antennule with small knob terminally; antenna bearing distal endopodal segment; labrum protruding ventrally; two pairs of biramous legs each with 2-segmented rami. Kokeshioides gen. nov. has the following combinations of features that distinguish it from other chondracanthid genera: body flattened, without lateral processes; cephalothorax much wider than long, with paired anterolateral and posterolateral lobes, folded ventrally; the very posteriormost part of the first pedigerous somite contributes to the neck; mandible elongate; legs unique, heavily sclerotized, represented by two pairs of acutely pointed processes.
Conclusion
With the addition of two new genera presently reported, the family Chondracanthidae currently includes 52 valid genera. Among the described genera Avatar gen. nov. seems to be very primitive, while Kokeshioides gen. nov. is highly advanced. The deduced evolutionary history of chondracanthid genera is also discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Studies on parasitic copepods infecting deep-sea fishes are still rare in comparison with those on shallow-water taxa. Deep-sea parasitic copepods are highly restricted to some genera belonging to the following families: Pennellidae Burmeister, 1835, Chondracanthidae Milne Edwards, 1840, Sphyriidae Wilson, 1919, Hyponeoidae Heegaard, 1962, and Hatschekiidae Kabata, 1979 (see Table 1 in Boxshall) [1].
The Chondracanthidae is one of the most speciose copepod families, that utilize fishes as hosts accommodating nearly 192 species in 50 valid genera [2]. Among them, 27 genera are monotypic and only two genera have more than ten species, Acanthochondria Oakley, 1930 with 54 valid species, and Chondracanthus Delaroche, 1811 with 41 valid species [2,3,4,5,6]. Molecular analyses are absolutely needed to confirm the validity of monotypic genera.
The documentation of the parasitic copepod fauna in Japanese waters began with the significant contributions by Yamaguti [7], Yamaguti and Yamasu [8], Wilson [9, 10], Shiino [11, 12] and Izawa [13], followed by Ohtsuka et al. [14, 15], Uyeno and Nagasawa [16] and Nagasawa et al. [17]. The family Chondracanthidae is comparatively well documented in Japanese waters, with 48 valid species in 20 genera (see Nagasawa et al. [17]).
In the presently reported study, we describe two new genera and species collected from two species of deep-sea fishes in Suruga Bay, Japan.
Materials and Methods
Specimens of chondracanthids were collected from the buccal cavity of two species of deep-sea fishes (fish hosts were frozen), Chaunax abei Le Danois, 1978 (Lophiiformes: Chaunacidae) and Setarches longimanus (Alcock, 1894) (Perciformes: Setarchidae), caught at a depth of 212 m in Suruga Bay, Japan (34°37′48.87″ N, 138°43′2.958″ E). Methods for preservation, dissection, mounting, and drawing of appendages were according to the techniques described in Aneesh et al. [18,19,20,21]. The specimens were microphotographed using Olympus microscopes (Olympus SZX7 and Olympus Bx50, Olympus Co., Ltd.) and image-capturing software (DP2-SAL, Olympus Co., Ltd). Total body length was measured (without egg sacs) from the anterior margin of the cephalothorax to the distal end of the caudal rami. Drawings were digital-inked using Adobe Illustrator and a WACOM CTL-472/K0-c drawing pad. Morphological terminology follows Huys and Boxshall [22]. The taxonomy and nomenclature of host fishes were adopted from Catalogue of Fishes [23] and FishBase [24]. The type material is deposited in the National Museum of Nature and Science, Tsukuba, Japan.
Taxonomy
-
Order Cyclopoida Burmeister, 1834
-
Family Chondracanthidae Milne Edwards, 1840
Genus Avatar gen. nov.
Type species: Avatar nishidai gen. et sp. nov. by original designation.
Diagnosis based on the adult female (bold = key features). Body small, flattened. Cephalothorax and first pedigerous somite fused forming cephalothorax. Cephalothorax, slightly wider than long with anterior pair of large and posterior pair of small lobes, and two pairs of ventro-lateral processes; latter processes located at level of mouthparts. The very posterior most part of the first pedigerous somite contributes to the neck. Third and fourth pedigerous somites fused into cylindrical trunk, bearing two pairs of blunt proximal fusiform processes. Antennule with large, fleshy, basal and terminal portion with few small elements. Antenna uncinate, distal endopodal segment represented by atrophied tip of antenna, a slender segment with five elements. Labrum protruding ventrally. Mandible falcate and bilaterally denticulated. Maxillule armed with two elements. Maxilla 2-segmented, terminal segment curved, slender, and with attenuated process bearing row of teeth. Maxilliped 3-segmented, armature of the typical chondracanthid type. Two pairs of biramous legs, each with 2-segmented rami. Other legs absent. Genitoabdomen with paired genital apertures on ventral surface. Caudal rami small, bearing one long and three short elements. Egg sac multiseriate.
Etymology: The generic name is derived from a world-famous epic science fiction film, James Cameron’s “Avatar”, in which the dragon-like aerial predator “Mountain Banshee” with two pairs of wings reminds us of the present new taxon with two pairs of lateral processes on the trunk. Gender feminine.
Remarks: Avatar can readily be separated from all other known chondracanthid genera by the following combination of features: (1) cephalothorax, slightly wider than long with anterior pair of large and posterior pair of small lobes, and two pairs of lateral processes; (2) posteriormost part of the first pedigerous somite contributes to the neck; (3) cylindrical trunk with two pairs of blunt, proximally fusiform processes; (4) antennule with small terminal knob; (5) antenna distal endopodal segment represented by the atrophied tip, slender segment with three apical elements; (6) labrum protruding ventrally; (7) two pairs of biramous legs, each with 2-segmented rami.
The presence of biramous legs 1 and 2, each with 2-segmented rami and an accessory lobe on the antenna indicates that Avatar gen. nov. resembles other chondracanthid genera such as Blias Krøyer, 1863, Diocus Krøyer, 1863, Humphreysia Leigh-Sharpe, 1934, Immanthe Leigh-Sharpe, 1934, and Juanettia Wilson, 1921 (see Table 1). The genus Blias differs from Avatar gen. nov. by (1) terminal antennary segment trifurcate (vs. uncinate antenna with accessory process in Avatar gen. nov.), (2) body processes absent (vs. cephalic and trunk processes present), and (3) legs biramous, unsegmented, each with a lobate protopod and rami (vs. biramous legs present with distinctly 2-segmented rami). Diocus can be distinguished from Avatar gen. nov. by (1) antennary segment T-shaped (vs. uncinate antenna with accessory processes), and (2) biramous legs without clear segmentation (vs. rami distinctly 2-segmented). The monotypic genus Humphreysia differs from Avatar gen. nov. by (1) trunk processes absent (vs. present), and (2) only one pair of biramous, unsegmented legs (vs. two pairs). Another monotypic genus Immanthe differs from Avatar gen. nov. by (1) trunk processes absent (vs. present), and (2) rami of both legs with endopod smaller than exopod (vs. both rami equal in size). The new genus Avatar is most closely related to the relatively primitive chondracanthid genus Juanettia, but both can be separated from each other by the number of biramous legs and their segmentation: only one pair of biramous, 1-segmented legs in Juanettia (vs. two pairs with 2-segmented rami).
Avatar nishidai gen. et sp. nov. (Figs. 1, 2, 3, 4, 5)
Material examined: Holotype—1 ♀ (3.3 mm) (Reg. No. NSMT-Cr 31607), from Chaunax abei collected at a depth of 212 m off Suruga Bay, Japan (34°37′48.87″ N, 138°43′2.958″ E); coll. Y. Nishida and S. Ohtsuka on 20 October 2022.
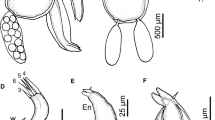
Description of holotype female: Body, small, flattened, 2.8 times as long as wide (lateral processes not included), total body length 3.3 mm. Cephalothorax and first pedigerous somite fused forming cephalothorax. Cephalothorax, nearly as long as wide with anterior pair of large and posterior pair of small lobes, and two pairs of ventrolateral processes, posterior one located on either side of mouth appendages. Posteriormost part of the first pedigerous somite contributes to the neck. Third and fourth pedigerous somites fused forming cylindrical trunk, bearing two pairs of blunt, proximally fusiform processes with narrow stalk basally (one pair at proximo-lateral corner and other at disto-lateral corner, both directed postero-laterally). Genitoabdomen about twice wider than long. Caudal rami 1.2 times as long as wide, bearing one long spiniform and three short setiform elements. Egg sac multiseriate, elongate, not coiled (Fig. 1A); number of eggs in right and left egg sacs approximately 450 and 580, respectively.
Antennule (Figs. 1B, 3B, C, 5A) large, fleshy, swollen, with few small elements and small knob terminally. Antenna (Figs. 1C, 3D, 5A, B) uncinate, 3-segmented, with coxa and basis fused to form coxobasis and 2-segmented endopod, proximal endopodal segment typically produced into powerful, curved claw while distal endopodal segment represented by slender segment with five elements (Fig. 3E). Labrum protruding (Fig. 3A). Mandible (Fig. 3F) represented by falcate blade, bilaterally denticulated. Maxillule (Fig. 3G) vestigial, lobate, tipped with two apical spinules. Maxilla (Fig. 3H) 2-segmented; syncoxa unarmed; basis curved, slender, and attenuated process bearing row of teeth. Maxilliped (Fig. 3I) 3-segmented, armature of typical chondracanthid type; first segment robust, unarmed; second segment with a patch of minute spinules on inner edge; distal segment, small, ending in curved claw-like structure with small, subterminal hooklet on inner surface.
Two pairs of biramous legs (Fig. 5C); protopod unarmed; exopod and endopod 2-segmented, armed with setae. Other legs absent. Leg 1 (Fig. 4A), located near maxilliped; first exopodal segment with an outer seta; second segment with nine setae along free margin; first endopodal segment unarmed; second segment with seven setae along free margin. Leg 2 (Fig. 4B), located in neck region; first exopodal segment with an inner seta; second segment with four setae along distal margin; first endopodal segment unarmed; second segment with five setae along distal margin.
-
Male: Unknown.
-
Color: White (before fixation).
-
Host: Known only from Chaunax abei Le Danois, 1978 (Chaunacidae), the type host.
-
Distribution: Known only from the type locality.
-
Etymology: The specific name of the new species, ‘nishidai’, is dedicated to Mr. Yusuke Nishida (Hiroshima University) who found this enigmatic chondracanthid in the Suruga Bay, Japan. It is a noun in the genitive case.
Genus Kokeshioides gen. nov.
Type species: Kokeshioides surugaensis gen. et sp. nov. by original designation.
Diagnosis based on the adult female (bold = key features). Body small, flattened. Cephalothorax fused with first pedigerous somite forming cephalothorax. Cephalothorax much wider than long, with lobate anterior and posterior corners, folded ventrally. Posteriormost part of the first pedigerous somite contributes to the neck. Third and fourth pedigerous somites fused forming shield-like trunk, without lateral processes; trunk anteriorly narrower at transition to neck. Antennule fleshy, swollen, with many spinules. Antenna uncinate, without accessory process. Mandible elongate, falcate and bilaterally denticulate. Maxillule armed with one element. Maxilla 2-segmented; basis curved, slender, with attenuate process bearing row of teeth. Maxilliped 3-segmented, first segment robust, unarmed; second segment with a patch of minute spinules on inner edge; distal segment, small, ending in curved claw-like structure with small, subterminal hooklet on inner surface. Legs 1 and 2, represented by pairs of acutely pointed processes. Other legs absent. Genital double-somite, highly reduced with paired genital apertures on ventral surface. Caudal rami bearing 1 long and 3 short setae. Egg sac unknown.
Etymology: The generic name is derived from a Japanese traditional wooden toy called “Kokeshi” and the Latin suffix -oides meaning “like”. Gender masculine.
Remarks: The genus Kokeshioides gen. nov. is clearly distinguishable from all other known chondracanthid genera by the following combination of features: (1) body flattened, without any processess; (2) cephalothorax, much wider than long, with lobate corners, folded ventrally. (3) posteriormost part of the first pedigerous somite contributes to the neck; (4) mandible elongate; (5) legs 1 and 2 unique, represented by paired acutely pointed processes.
The presence of two pairs of highly modified spiniform legs makes Kokeshioides gen. nov. unique among all known chondracanthid genera. The genera Humphreysia and Immanthe show some similarity to Kokeshioides gen. nov., especially in general body shape and the absence of trunk processes (see Table 1). In addition to the key diagnostic features of Kokeshioides gen. nov. (highly modified spiniform legs), Humphreysia can be differentiated from the latter by (1) antenna with vestigial tip (second endopodal segment) in Humphreysia (vs. without), (2) cephalic processes present in Humphreysia (vs. absent); (3) only one pair of biramous segmented legs with armature in Humphreysia (vs. two pairs of highly modified legs). Immanthe can be separated from the Kokeshioides by (1) presence of one pair of cephalic processes (vs. absent), and (2) rami of both legs, endopod smaller than exopod in Immanthe (vs. legs spiniform).
Kokeshioides surugaensis gen. et sp. nov. (Figs. 6, 7, 8, 9, 10, 11)
Material examined: Holotype—1 ♀ (4.1 mm) (Reg. No. NSMT-Cr 31608), from Setarches longimanus (Alcock, 1894) (Setarchidae), collected at a depth of 212 m off Suruga Bay, Japan (34°37′48.87″ N, 138°43′2.958″ E); coll. S. Ohtsuka on 20 October 2022. Paratype—1 ♀ (4.0 mm) (Reg. No.NSMT-Cr 31609); same data as holotype.
Description of holotype female. Body (Figs. 6, 7, 8), small, flattened, 2.7 times as long as wide, widest in posterior part, total body length, 4.0–4.1 mm. Cephalothorax consisting of cephalothorax and first pedigerous somite. Cephalothorax, 1.4 times as wide as long, with lobate extensions at anterior and posterior corners, folded ventrally; lateral surface of dorsal shield armed with many spinules. Posteriormost part of the first pedigerous somite contributes to the neck. Third and fourth pedigerous somites fused, forming trunk, without lateral processes as expressed in Avatar nishidai gen. et sp. nov.; trunk tapering anteriorly towards transition with neck (Fig. 8). Genitoabdomen (Figs. 8A, 10G–I, 11A, B) highly reduced with paired genital apertures on ventral surface, carrying pair of ventrally folded caudal rami, bearing 1 long and 3 short setae. Egg sacs not observed.
Antennule (Figs. 7C, D, 8, 9A, B, D) fleshy, with large, swollen basal portion and small setose terminal part. Antenna (Figs. 7E, 8A, C, 9B) uncinate, 2-segmented, basis without any process; endopod modified into medially-curved hook. Mandible (Fig. 10A) elongate, falcate blade, bilaterally denticulate. Maxillule (Fig. 10B) vestigial, lobate, armed with one minute element. Maxilla (Fig. 10C) 2-segmented, terminal segment represented by curved, slender, attenuated process bearing row of teeth. Maxilliped (Fig. 10D) 3-segmented, first segment robust, unarmed; second segment with a patch of minute spinules on inner edge; distal segment, small, ending in curved claw-like structure with small, subterminal hooklet on inner surface.
Two pairs of biramous, highly modified legs (Figs. 6, 7B, 8, 10E, F, 11C, D; basis broad, plate-like, surface slightly rough, with patches of minute pustules. Rami modified into acutely pointed processes, movable at base (arrowed in Fig. 11C, D). Other legs absent. Leg 1 (Figs. 10E, 11C, D) located posterior to mouth appendages, basis unarmed; exopod, shorter than endopod (0.6 times as long as endopod). Leg 2 (Figs. 10F, 11C, D) located in neck region, basis slightly narrower than that of leg 1; exopod shorter than endopod (0.4 times as long as endopod).
-
Male: Unknown.
-
Color: White (before fixation).
-
Host: Known only from Setarches longimanus (Alcock, 1894) (Setarchidae).
-
Distribution: Known only from the type locality.
-
Etymology: The specific name is derived from the type locality, Suruga Bay, Japan. It is in the nominative singular, gender masculine.
Discussion
The family Chondracanthidae was thoroughly revised by Ho [25], who excluded 12 old genera and provided a key to the 30 valid genera recognized at that time. Twelve genera were added subsequently by various authors which Ho [26] included in his revised key. Three genera were subsequently synonymised [27]. After the revision of Ho [26] eight new genera have been established, the latest addition of which being the discovery of Ttetaloia Uyeno and Nagasawa, 2012 from Japan and the three previously excluded genera (viz. Immanthe Leigh-Sharpe, 1934, Lernaeosolea Wilson, 1944, and Pharodes Wilson, 1935) were restored, based on unique combinations of generic characters [2, 6, 26, 27]. With the addition of the two new genera in the presently reported study, Avatar gen. nov. and Kokeshioides gen. nov., the family Chondracanthidae currently includes 52 valid genera.
Many chondracanthid genera possess usually paired ventrolateral processes on the 3 or 4 pedigerous somites; for instance, members of the genus Chondracanthus have well-developed trunk processes, and their number may vary in different species. Our observation of Chondracanthus kabatai Aneesh, Helna, and Kumar, 2020 reveals that these ventrolateral processes contain oviducts, providing space for the maturing oocytes, and thereby enhancing the fecundity. The majority of chondracanthid genera possess an uncinate, strongly prehensile antenna; few others have a bifurcate, trifurcate or clavate (non-uncinate type) antenna and some relatively primitive chondracanthid genera such as Avatar gen. nov., Humphreysia, Immanthe, Juanettia etc., possess the distal endopodal segment, represented by the atrophied tip of the antenna as a slender segment with 2–6 apical elements [28, 29]. The structure seems to have been lost during evolution. For instance, it is totally absent in highly advanced genera such as Acanthochondria, Chondracanthus, Kokeshioides gen. nov. etc. In contrast, the morphology of the mouth appendages seems to be relatively constant within the family, suggesting that most species share a similar feeding mode [21, 25, 26].
Various evolutionary trends can be observed in the development of legs, especially legs 1 and 2 in the family. Legs are highly modified or rudimentary in females chondracanthids and vary in different genera: only one biramous 2-segmented leg is present in primitive chondracanthids such as Juanettia, Auchenochondria Dojiri and Perkins, 1979 and Humphreysia; two pairs of unmodified, biramous legs are present in Avatar gen. nov.; uniramous or unilobate or bilobate legs are present in remaining chondracanthid genera; and legs are totally absent in Apodochondria Ho and Dojiri, 1988 (see Ho [25, 26]). The highly modified, spiniform legs in Kokeshioides gen. nov. can be considered the most advanced state in the Chondracanthidae. The segmentation of the legs is clear in the relatively primitive genera such as Cryptochondria Izawa, 1971, Juanettia and Rhynchochondria Ho, 1967. Between these most advanced and primitive conditions, a wide variety of intermediate conditions can be seen: the segmentation of the rami of the legs is lost in some species of Protochondria Ho, 1970, reduction in size of the rami of legs in Brachiochondrites Markevich, 1940, Blias, Chondracanthodes Wilson, 1932 or just in the size of the endopod in Humphreysia (see Kabata [30]). Along with the size reduction, the number and size of setae are also reduced in Pseudacanthocanthopsis Yamaguti and Yamasu, 1959 and some species of Protochondracanthus Kirtisinghe, 1950, and seta are totally absent in many advanced genera in Blias, Chondracanthus, Chondracanthodes, Pseudacanthocanthopsis, Pseudoblias Heegaard, 1962, Acanthochondria, and Acanthochondrites Oakley, 1930. The legs will be either totally absent or highly modified, represented by setae or spiniform processes during the course of evolution (in Kokeshioides gen. nov.) (see Kabata [30]). Throughout the evolution of the family, legs might have been reduced into functionless elements or modified new functional grasping organs.
Conclusions
New material collected from two different species of deep-sea fishes of Suruga Bay, Japan were found to be different from all other known chondracanthid genera, and based on the clear morphological features we described two new monotypic genera. Accordingly, we described Avatar nishidai gen. et sp. nov. from Chaunax abei Le Danois, 1978 (Chaunacidae) and Kokeshioides surugaensis gen. et sp. nov. from Setarches longimanus (Alcock, 1894) (Setarchidae). By the description of two new genera in the presently reported study, the family Chondracanthidae currently includes 52 valid genera. Among the described genera Avatar gen. nov. seems to be very primitive, while Kokeshioides gen. nov. is highly advanced. The deduced evolutionary history of chondracanthid genera is also discussed in the present paper.
Data Availability
The type materials are deposited in the National Museum of Nature and Science, Tsukuba, Japan.
References
Boxshall GA (1998) Host specificity in copepod parasites of deep-sea fishes. J Mar Syst 15:215–223. https://doi.org/10.1016/S0924-7963(97)00058-4
Walter C, Boxshall GA (2023) World of copepods database. Accessed at http://www.marinespecies.org/copepoda on 06 June 2019
Tang D, Andrews M, Cobcroft JM (2007) The first chondracanthid (Copepoda: Cyclopoida) reported from cultured finfish, with a revised key to the species of Chondracanthus. J Parasitol 93:788–795. https://doi.org/10.1645/GE-1121R.1
Braicovich PE, Lanfranchi AL, Incorvaia IS, Timi JT (2013) Chondracanthid copepod parasites of dories (Zeiformes: Zeidae) with the description of a new species of Chondracanthus from waters off northern Argentina. Folia Parasitol 60:359–364. https://doi.org/10.14411/fp.2013.037
Gómez S, Aguirre-Villaseñor H, Morales-Serna FN (2018) A new species of Chondracanthus (Cyclopoida: Chondracanthidae) parasitic on deep-sea Dibranchus spongiosa (Lophiiformes: Ogcocephalidae) from the eastern central Pacific. Acta Parasitol 63:375–385. https://doi.org/10.1515/ap-2018-0043
Aneesh PT, Helna AK, BijuKumar A (2020) New species of Acanthochondria Oakley, 1930 and Chondracanthus Delaroche, 1811 (Copepoda: Poecilostomatoida: Chondracanthidae) parasitizing marine fishes from Indian coast. Nauplius 28:e2020014. https://doi.org/10.1590/2358-2936e2020014
Yamaguti S (1939) Parasitic copepods from fishes of Japan. Part 6. Lernaeopodoida, 1. Volume Jubilare pro Prof. Sadao Yoshida 2:529–578, pls 34–58 (iii-1939)
Yamaguti S, Yamasu T (1959) Parasitic copepods from fishes of Japan with description of 26 new species and remarks on two known species. Biol J Okayama Univ 5(3–4):89–165, pls 1–14 (ix-1959)
Wilson CB (1935) Parasitic copepods from the Dry Tortugas. Papers of the Tortugas Laboratory 29 (12):329–347, pls 1–6 (Publs Carnegie Inst Wash 452)
Wilson CB (1944) Parasitic copepods in the United States National Museum. Proc US Nat Mus 94(3177):529–582, plates 20–34
Shiino SM (1955) Copepods parasitic on Japanese fishes, 9. Family Chondracanthidae, subfamily Chondracanthinae. Rep Fac Fish Pref Univ Mie 2:70–111
Shiino SM (1960) Two new parasitic copepods belonging to a new genus Prochondracanthopsis (Chondracanthidae). Rep Fac Fish Pref Univ Mie 3:518–526
Izawa K (1975) A new and a known Chondracanthid copepods parasitic on fishes from Tanabe Bay. Annot Zool Jpn 48(2):108–118, figs 1–32 (20-vi-1975)
Ohtsuka S, Boxshall GA, Harada S (2005) A new genus and species of nicothoid copepod (Crustacea: Copepoda: Siphonostomatoida) parasitic on the mysid Siriella okadai Ii from off Japan. Syst Parasitol 62:65–81. https://doi.org/10.1007/s11230-005-5483-x
Ohtsuka S, Dhugal JL, Izawa K (2018) A new genus and species of the family Pennellidae (Copepoda, Siphonostomatoida) infecting the Pacific viperfish Chauliodus macouni. Parasite 25(6):1–10. https://doi.org/10.1051/parasite/2018003
Uyeno D, Nagasawa K (2012) Ttetaloia hoshinoi, a new genus and species of chondracanthid copepod (Poecilostomatoida) parasitic on triplefins (Actinopterygii: Tripterygiidae) from Japanese waters. Zoosymposia 8:39–48
Nagasawa K, Uyeno D, Ho J-S (2013) A checklist of copepods of the family Chondracanthidae (Poecilostomatoida) from fishes in Japanese waters (1918–2013). Biosphere Science (Japan) 52:117–143
Aneesh PT, Helna AK, Kumar BA, Venmathi Maran BA (2018) Redescription of Lernaeenicus stromatei Gnanamuthu, 1953 (Copepoda: Siphonostomatida: Pennellidae) infesting the Black Pomfret Parastromateus niger (Bloch) from Indian waters. Zootaxa 4482(2):375–382. https://doi.org/10.11646/zootaxa.4482.2.9
Aneesh PT, Helna AK, Kumar AB, Maran BAV (2021) A new species of parasitic copepod of the genus Lernaeenicus Le Sueur, 1824 (Siphonostomatoida: Pennellidae) from the Torpedo scad Megalaspis cordyla (Linnaeus) off Kerala coast, India. Mar Biol Res 17:1–11. https://doi.org/10.1080/17451000.2021.1887498
Aneesh PT, Helna AK, Prabhakaran MP, Ravinesh R, Kumar AB (2021) Complementary description and range extension of an unusual caligid copepod Anchicaligus nautili (Willey, 1896) (Copepoda: Siphonostomatoida) parasitizing the endangered deep-sea cephalopod Nautilus pompilius Linnaeus, 1758 from the Indian Ocean. Thalassas 37:757–766. https://doi.org/10.1007/s41208-021-00331-2
Aneesh PT, Helna AK, Bijukumar A, Maran BAV (2023) Proposal of a new family for Hirodai ohtsukai gen et sp nov (Crustacea: Copepoda) infesting Uranoscopus guttatus Cuvier, 1829 (Perciformes: Uranoscopidae) from the southwest coast of India. J Nat Hist 57(33–36):1495–1515. https://doi.org/10.1080/00222933.2023.2259556
Huys R, Boxshall GA (1991) Copepod evolution. Ray Society, London
Fricke R, Eschmeyer WN, van der Laan R (eds) (2023) Catalog of fishes: genera, species, references. Electronic version Accessed 08 Feb 2023. http://research.calacademy.org/research/ichthyology/catalog/fishcatmain.asp
Froese R, Pauly D (eds) (2023) FishBase. World Wide Web electronic publication, version (09/2009). Accessed 08 Feb 2023. www.fishbase.org
Ho J-S (1970) Revision of the genera of the Chondracanthidae, a copepod family parasitic on marine fishes. Beaufortia 229:105–218
Ho J-S, Kim I-H (1994) Chondracanthid copepods (Poecilostomatoida) parasitic on Japanese deep-sea fishes, with a key to the genera of the Chondracanthidae. J Nat Hist 28:505–517. https://doi.org/10.1080/00222939400770231
Østergaard P, Boxshall GA, Quicke DLJ (2003) Phylogeny within the Chondracanthidae (Poecilostomatoida, Copepoda). Zool Scr 32:299–319. https://doi.org/10.1046/j.1463-6409.2003.00113.x
Ho JS (1984) Accessory antennule and the origin of the Chondracanthidae (Poecilostomatoida). In: Vervoort W, Vaupel Klein von JC (eds) Studies on Copepoda II. Proceedings of the first international conference on Copepoda, Amsterdam, The Netherlands, 24–28 August 1981. Crustaceana, Supplement 7:242–248
Huys R, Llewellyn-Hughes J, Olson PD, Nagasawa K (2006) Small subunit rDNA and Bayesian inference reveal Pectenophilus ornatus (Copepoda incertae sedis) as highly transformed Mytilicolidae, and support assignment of Chondracanthidae and Xarifiidae to Lichomolgoidea (Cyclopoida). Biol J Lin Soc 87(3):403–425
Kabata Z (1979) Parasitic Copepoda of British fishes. The Ray Society, London, 152:i-xii, 1–468, figs 1–203
Acknowledgements
This study was partially supported by grants-in-aid from the Japan Society of Promotion of Science (KAKENHI No. 19H03032, awarded to SO; JSPS Bilateral Partnership Program, No. JPJSBP120209924, awarded to SO). We thankfully acknowledge Prof. Jun Nishikawa, Tokai University for his valuable support.
Funding
Open Access funding provided by Hiroshima University.
Author information
Authors and Affiliations
Contributions
PTA, SO, and YK conducted the fieldwork. PTA, SO, and AKH, conceived and designed the research, and critically reviewed it to improve the quality of the manuscript. PTA and AKH worked on identification, illustrations, and pictures and prepared the draft of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Coflict of Interests
All authors declare that they have no competing interests. No potential conflict of interest was reported by the authors.
Consent for Publication
All the authors consent to the publication of this manuscript.
Ethical Approval
The specimen is not under the listed category of experimental animals, which needs ethics approval.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Aneesh, P.T., Ohtsuka, S., Kondo, Y. et al. Two New Genera and Species of the Parasitic Copepod Family Chondracanthidae Milne Edwards, 1840 (Copepoda: Cyclopoida) from Deep-Sea Fishes Off Suruga Bay, Japan. Acta Parasit. 69, 874–888 (2024). https://doi.org/10.1007/s11686-024-00820-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11686-024-00820-3