Abstract
Introduction
The lobster cockroach Nauphoeta cinerea (N. cinerea) is indicated as a promising non-mammalian model, because it presents behavioral and biochemical alterations also observed in conventional models. In this research, we identified and characterized the distribution of protozoa that inhabit the digestive system (DS) of N. cinerea cockroaches.
Methods
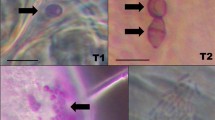
The adult specimens of N. cinerea used in this study (n = 32) were obtained at the Federal University of Santa Maria, dissected and had their visceral contents observed in bright-field microscopy without staining and after application of lugol, Ziehl–Neelsen staining, EA36 trichrome and simulated dark-field microscopy with application of nankin ink. The presence of protozoa in different portions of the DS was semi-quantified by a system of crosses (+).
Results
The main taxa observed were: amoebas (Archaemebae:Entamoebida), gregarins (Apicomplexa:Eugregarinide), coccidia (Apicomplexa:Eucoccidiorida), kinetoplastids (Kinetoplastea:Kinetoplastida) and oxymonads (Preaxostyla:Oxymonadida). The highest prevalence of amoebas and gregarines was observed in the medial portion of the DS, while for the other groups, this was seen in the final portion, and in the case of coccidia, such prevalence was specially evidenced by the alcohol-acid coloration. In the present work, the great biological diversity that exists in the microbiota of the digestive system of Nauphoeta cinerea was demonstrated, being possible to find several pathogenic species for humans such as Entamoeba histolytica/dispar/moshkovskii, Cryptosporidium sp. and Cyclospora cayetanensis. There is still a lot to know about the interactions between endocommensal protozoa and their respective invertebrate hosts, so the best way to clarify such relationships is through molecular and genetic test.





Similar content being viewed by others
References
Rao X, Liao Q, Pan T, Li S, Zhang X, Zhu S, Lin Z, Qiu Y, Liu J (2014) Retrospect and prospect of Lophomonas blattarum infections and lophomoniasis reported in China. Open Access Libr 1(9):1. https://doi.org/10.4236/oalib.1101121
Ding Q, Shen K (2021) Pulmonary infection with Lophomonas blattarum. Indian J Pediatr 88(1):23–27. https://doi.org/10.1007/s12098-020-03311-1
Pufpaff M, McCann DP (2021) The growth of cockroach farming in china. in: doing good business in China: case studies. In: International Business Ethics (pp. 129–134). World Scientific. https://doi.org/10.1142/9789811233654_0015
Ukoroije RB, Bawo DS (2020) Cockroach (Periplaneta americana): Nutritional value as food and feed for man and livestock. Asian Food Sci J. https://doi.org/10.9734/afsj/2020/v15i230150
Tang Q, Bourguignon T, Willenmse L, De Coninck E, Evans T (2019) Global spread of the German cockroach, Blattella germanica. Biol Invas 21(3):693–707. https://doi.org/10.1007/s10530-018-1865-2
Tsai, YH, Cahill KM (1970) Parasites of the German cockroach (Blattella germanica L.) in New York city. J. Parasitol: 375–377.
Martínez-Girón R, Martínez-Torre C, van Woerden HC (2017) The prevalence of protozoa in the gut of German cockroaches (Blattella germanica) with special reference to Lophomonas blattarum. Parasitol Res 116(11):3205–3210. https://doi.org/10.1007/s00436-017-5640-6
de Oliveira LM, da Silva Lucas AJ, Cadaval CL, Mellado MS (2017) Bread enriched with flour from cinereous cockroach (Nauphoeta cinerea). Innov Food Sci Emerg Technol 44:30–35. https://doi.org/10.1016/j.ifset.2017.08.015
Carvalho TS, Saad CE, Alvarenga RR, Oliveira EA, Carvalho MC, Ramos LG, Ferreira LG, Gonçalves TM, Costa DV, Zangeronimo MG (2019) Inclusion of Madagascar cockroach (Gromphadorhina portentosa) meal in the diet of cockatiels (Nymphicus hollandicus) in captivity: influences on offspring development. Res Vet Sci 126:89–93. https://doi.org/10.1016/j.rvsc.2019.08.016
Al-Mayali HMH, Al-Yaqoobi MSM (2010) Parasites of Cockroach Periplaneta americana (L.) in Al-Diwaniya province, Iraq. J Thi-Qar Sci 2(3).
Rodrigues NR, Nunes MEM, Silva DGC, Zemolin APP, Meinerz DF, Cruz LC, Pereira AB, Rocha JBT, Posser T, Franco JL (2013) Is the lobster cockroach Nauphoeta cinerea a valuable model for evaluating mercury induced oxidative stress? Chemosphere 92(9):1177–1182. https://doi.org/10.1016/j.chemosphere.2013.01.084
Adedara IA, Awogbindin IO, Afolabi BA, Ajayi BO, Rocha JB, Farombi EO (2020) Hazardous impact of diclofenac exposure on the behavior and antioxidant defense system in Nauphoeta cinerea. Environ Pollut 265:115053. https://doi.org/10.1016/j.envpol.2020.115053
Leal A, Karnopp E, Barreto YC, Oliveira RS, Rosa ME, Borges BT, Goulart FL, Souza VQ, Laikowski MM, Sidnei S, Vinadé L, da Rocha JBT, Dal Belo CA (2020) The insecticidal activity of Rhinella schneideri (Werner, 1894) paratoid secretion in Nauphoeta cinerea cocroaches. Toxins 12(10):630. https://doi.org/10.3390/toxins12100630
Carrasco HJ, Segovia M, Londoño JC, Ortegoza J, Rodríguez M, Martínez CE (2014) Panstrongylus geniculatus and four other species of triatomine bug involved in the Trypanosoma cruzi enzootic cycle: high risk factors for Chagas’ disease transmission in the Metropolitan District of Caracas Venezuela. Parasit Vect 7(1):1–15
Alexandre J, Sadlova J, Lestinova T, Vojtkova B, Jancarova M, Podesvova L, Yurchenko V, Dantas-Torres F, Brandão-Filho SP, Volf P (2020) Experimental infections and co-infections with Leishmania braziliensis and Leishmania infantum in two sand fly species, Lutzomyia migonei and Lutzomyia longipalpis. Sci Rep 10(1):1–8
Chamavit, P, Sahaisook P, Niamnuy N (2011) The majority of cockroaches from the Samutprakarn province of Thailand are carriers of parasitic organisms. EXCLI J 10: 218. https://doi.org/10.17877/DE290R-3250
Tan ZN, Wong WK, Nik Zairi Z, Abdullah B, Rahmah N, Zeehaida M, Rumaizi S, Lalitha P, Tan GC, Olivos-Garcia A, Lim BH (2010) Identification of Entamoeba histolytica trophozoites in fresh stool sample: comparison of three staining techniques and study on the viability period of the trophozoites. Trop Biomed 27(1):79–88
Aghamolaie S, Rostami A, Fallahi S, Tahvildar Biderouni F, Haghighi A, Salehi N (2016) Evaluation of modified Ziehl-Neelsen, direct fluorescent-antibody and PCR assay for detection of Cryptosporidium spp. in children faecal specimens. J Parasit Dis 40(3):958–963. https://doi.org/10.1007/s12639-014-0614-4
Pereira PS, Costa AR, de Oliveira TJS, Oliveira CVB, de Lima MDCA, de Oliveira JF, Kim B, Coutinho HDM, Duarte AE, Kamdem JP, da Silva TG (2022) Neurolocomotor behavior and oxidative stress markers of Thiazole and thiazolidinedione derivatives against Nauphoeta cinerea. Antioxidants 11(2):420
Bell WJ (1981) The laboratory cockroach. Experiments in cockroach anatomy, physiology and behavior. Springer. https://doi.org/10.1007/978-94-011-9726-7
Godoy IA, Fontana LC, Cordeiro EF, Khouri S, Ferreira-Strixino J (2014) Saúde da mulher: estudo citológico e microbiológico do trato geniturinário de pacientes do centro de práticas supervisionadas da UNIVAP. Revista Univap 20(35):5–14
de Sá JM, Colombo TE (2018) Infecção pelo Papilomavírus humano (HPV) em mulheres dos municípios de São José do Rio Preto e Olímpia de janeiro de 2015 até janeiro de 2016. J Health Sci Inst 36(2):99–104
Smith LE, Smallwood R, Macneil S (2010) A comparison of imaging methodologies for 3D tissue engineering. Microsc Res Tech 73(12):1123–1133. https://doi.org/10.1002/jemt.20859
Fisher Diagnostics (2021) Protocol Trichrome Stain. https://www.thermofisher.com/document-connect/document-connect.html?url=https%3A%2F%2Fassets.thermofisher.com%2FTFS-Assets%2FCDD%2Fmanuals%2FD19757~.pdf. Acessed: 10 December 2021.
Goggin CL, Lester RJG (1987) Occurrence of Perkinsus species (Protozoa, Apicomplexa) in bivalves from the Great Barrier Reef. Dis Aquat Org 3(2):113–117. https://doi.org/10.3354/dao003113
Archibald JM, Simpson AGB, Slamovits CH (2017) Handbook of the Protists (2 ed.). Springer. https://doi.org/10.1007/978-3-319-28149-0
CDC (2021) DPDx—Laboratory Identification of Parasites of Public Health Concern A-Z Index. https://www.cdc.gov/dpdx/az.html. Acessed: 10 December 2021.
Fakhri MH (2015) Phylogeny and diversity of Entamoeba in cockroaches, with an emphasis on Periplaneta americana. Dissertation, University of Arkansas.
Al-bayati NY, Al-Ubaidi AS, Al-Ubaidi IK (2011) Risks associated with cockroach Periplaneta americana as a transmitter of pathogen agents. DJM 1(1):91–97
Deere JR, Parsons MB, Lonsdorf EV, Lipende I, Kamenya S, Collins DA, Dominic AT, Gillespie TR (2019) Entamoeba histolytica infection in humans, chimpanzees and baboons in the Greater Gombe Ecosystem, Tanzania. Parasitology 146(9):1116–1122. https://doi.org/10.1017/s0031182018001397
Samie A, Mahlaule L, Mbati P, Nozaki T, ElBakri A (2020) Prevalence and distribution of Entamoeba species in a rural community in northern South Africa. Food Waterb Paras 18:e00076. https://doi.org/10.1016/j.fawpar.2020.e00076
Yanagawa Y, Nagata N, Yagita K, Watanabe K, Okubo H, Kikuchi Y, Gatanaga H, Oka S, Watanabe K (2021) Clinical features and gut microbiome of asymptomatic Entamoeba histolytica infection. Clin Infect Dis 73(9):e3163–e3171. https://doi.org/10.1093/cid/ciaa820
Clopton, RE, Hays, JJ (2006) Revision of the genus Protomagalhaensia and description of Protomagalhaensia wolfi n. comb. (Apicomplexa: Eugregarinida: Hirmocystidae) and Leidyana haasi n. comb. (Apicomplexa: Eugregarinida: Leidyanidae) parasitizing the lobster cockroach, Nauphoeta cinerea (Dictyoptera: Blaberidae). Comp Parasitol 73(2): 137–156. https://doi.org/10.1654/4241.1
Clopton RE (2009) Phylogenetic relationships, evolution, and systematic revision of the septate gregarines (Apicomplexa: Eugregarinorida: Septatorina). Comp Parasitol 76(2):167–190. https://doi.org/10.1654/4388.1
Clopton RE (2010) Protomagalhaensia cerastes n. sp. (Apicomplexa: Eugregarinida: Blabericolidae) parasitizing the pallid cockroach, Phoetalia pallida (Dictyoptera: Blaberidae). Comp. Parasitol. 77(2): 117–124. https://doi.org/10.1654/4443.1
Chalmers RM, Robinson G, Elwin K, Elson R (2019) Analysis of the Cryptosporidium spp. and gp60 subtypes linked to human outbreaks of cryptosporidiosis in England and Wales, 2009 to 2017. Paras Vect 12(1): 1–13. https://doi.org/10.1186/s13071-019-3354-6
Crestia J, Razakandrainibe R, Costa D, Damiani C, Totet A, Le Govic Y (2021) Seven shades of Cryptosporidium. Clin Microbiol Infect. https://doi.org/10.1016/j.cmi.2021.04.023
Zerpa R, Huicho L (1994) Childhood cryptosporidial diarrhea associated with identification of Cryptosporidium Sp. in the cockroach periplaneta Americana. Pediatr Infect Dis J 13(6): 546–548. https://doi.org/10.1097/00006454-199406000-00019
Shaposhnik EG, Abozaid S, Grossman T, Marva E, On A, Azrad M, Peretz A (2019) The prevalence of Cryptosporidium among children hospitalized because of gastrointestinal symptoms and the efficiency of diagnostic methods for Cryptosporidium. Am J Trop Med Hyg 101(1):160. https://doi.org/10.4269/ajtmh.19-0057
Masangkay FR (2019) Increased detection of Cryptosporidium and Cyclospora spp. oocysts in a major Philippine watershed following rainfall events. Asian J Biol Sci 8(3): 111. https://doi.org/10.5530/ajbls.2019.8.18
Erturk EY, Karaman U, Colak C, Direkel S, Arici YK (2021) Prevalence of Cyclospora cayetanensis and Cryptosporidium spp. children according to some variables. Medicine 10(2): 338–45. https://doi.org/10.5455/medscience.2020.11.238
CDC (2018) Multistate outbreak of cyclosporiasis linked to Del Monte fresh produce vegetable trays—United States. Atlanta, GA: US Department of Health and Human Services, CDC; 2018. https://www.cdc.gov/parasites/cyclosporiasis/outbreaks/2018/a-062018/index.html. Acessed: 10 December 2021.
Nguhiu PN, Wamae CN, Magambo JK, Mbuthia PG, Chai DC, Yole DS (2012) Gross and histopathological findings in Cercopithecus aethiops with experimental Cyclospora infection in Kenya. Pathol Lab Med Int. https://doi.org/10.2147/PLMI.S28142
Tsang OTY, Wong RWC, Lam BHS, Chan JMC, Tsang KY, Leung WS (2013) Cyclospora infection in a young woman with human immunodeficiency virus in Hong Kong: a case report. BMC Res Notes 6(1):1–5. https://doi.org/10.1186/1756-0500-6-521
Borghesan TC, Ferreira RC, Takata CS, Campaner M, Borda CC, Paiva F, Milder RV, Teixeira MMG, Camargo EP (2013) Molecular phylogenetic redefinition of Herpetomonas (Kinetoplastea, Trypanosomatidae), a genus of insect parasites associated with flies. Protist 164(1):129–152. https://doi.org/10.1016/j.protis.2012.06.001
Yurchenko V, Kostygov A, Havlová J, Grybchuk‐Ieremenko A, Ševčíková T, Lukeš J, Sevcik J, Votýpka J (2016) Diversity of trypanosomatids in cockroaches and the description of Herpetomonas tarakana sp. n. J Eukaryot Microbiol 63(2): 198–209. https://doi.org/10.1111/jeu.12268
Bamidele A, Abayomi A, Iyabo A, Giwa M (2019) Parasitic fauna, histopathological alterations, and organochlorine pesticides contamination in Chrysichthys nigrodigitatus (Lacepede, 1803) (Bagridae) from Lagos, Lagoon Nigeria. Sci Afr 5:e00130. https://doi.org/10.1016/j.sciaf.2019.e00130
Mendes PM, Lansac-Tôha FM, Meira BR, Oliveira FR, Velho LFM, Lansac-Tôha FA (2019) Heterotrophic flagellates (Amorpha and Diaphoretiches) in phytotelmata bromeliad (Bromeliaceae). Braz J Biol 80:648–660. https://doi.org/10.1590/1519-6984.218742
Treitli SC, Kotyk M, Yubuki N, Jirounková E, Vlasáková J, Smejkalová P, Sípek P, Čepičkab V, Hampl, V (2018) Molecular and morphological diversity of the oxymonad genera Monocercomonoides and Blattamonas gen. nov. Protist 169(5): 744–783. https://doi.org/10.1016/j.protis.2018.06.005
Funding
This research was funded by the National Research and Development Council (CNPq) through the granting of the scientific initiation grant process no. [133380/2021–1] established by the institutional grant program—02/2020 PIBIC/CNPq/URCA, of the Regional University of Cariri—URCA.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors have nothing to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Oliveira, C.V.B., Neves, D.H., de Souza Morais, E.E. et al. Identification and Semi-quantification of Protozoa from the Digestive System Microbiota of the Lobster Cockroach Nauphoeta cinerea Oliver, 1789 (Insecta:Blattaria). Acta Parasit. 67, 1186–1198 (2022). https://doi.org/10.1007/s11686-022-00570-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11686-022-00570-0