Abstract
Purpose
Babesiosis is one of the most important globally extended and quickly spreading tick-borne infections of dogs. Diagnosis of babesiosis in Sri Lanka is based on clinical signs followed by thin blood smears which could be error-prone due to undetected early infections, absence of clinical signs or low parasitemia. The present study investigated the prevalence of babesiosis in dogs presented to the Veterinary Teaching Hospital (VTH) at the Faculty of Veterinary Medicine and Animal Science, University of Peradeniya, for treatments, vaccinations, and regular check-ups, and compared the diagnosis methods of microscopy and molecular analysis.
Methods
Blood samples from dogs were collected from January to June 2019. First, Giemsa stained blood smears were prepared, and then the blood samples were subjected to PCR using genus-specific primers to amplify a 411–450 bp region in the 18S rRNA gene. Twenty samples from PCR amplified products were sequenced for species identification and phylogenetic analysis. Clinical signs of the dogs were noted down, and ticks were also collected from dogs if any.
Results
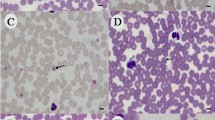
Results show a very high prevalence of canine babesiosis (78.6%) among the dogs brought to the VTH. The parasite was identified microscopically and genetically as Babesia gibsoni. A large percentage (66.7%) of infections was asymptomatic. Out of 42 blood samples, 19 (45.2%) were microscopically positive for babesiosis while 33 (78.6%) were PCR positive, showing a significant difference in the two methods of diagnosis (chi-square test, χ2 = 9.462, p = 0.002). Three tick species: Rhipicephalus haemaphysaloides, Haemaphysalis bispinosa, and Rhipicephalus sanguineus were found attached to the dogs.
Conclusion
This study shows a very high prevalence of canine babesiosis among dogs in the Kandy area. Most of these infections might go undetected if only microscopy was used to diagnose. An improved, rapid diagnostic method such as the novel, PCR-based point-of-care diagnostic method that detects very low parasitemia within 30 min is needed. Moreover, as most infected dogs did not show clinical signs, they may act as reservoirs of infection. The ability of asymptomatic dogs to spread babesiosis should be investigated.

Similar content being viewed by others
Data availability
Informed.
Code availability
Not applicable.
References
Kjemtrup AM, Kocan AA, Whitworth L, Meinkoth J, Birkenheuer AJ, Cummings J, Boudreaux MK, Stockham SL, Irizarry-Rovira A, Conrad PA (2000) There are at least three genetically distinct small piroplasms from dogs. Int J Parasitol 30:1501–1505. https://doi.org/10.1016/S0020-7519(00)00120-X
Bilić P, Kuleš J, Barić Rafaj R, Mrljak V (2018) Canine babesiosis: where do we stand? Acta Vet Brno 68:127–160. https://doi.org/10.2478/acve-2018-0011
Matijatko V, Torti M, Schetters TP (2012) Canine babesiosis in Europe: how many diseases? Trends Parasitol 28:99–105. https://doi.org/10.1016/j.pt.2011.11.003
Karbowiak G, Biernat B, Stańczak J, Werszko J, Szewczyk T, Sytykiewicz H (2018) The role of particular ticks developmental stages in the circulation of tick-borne pathogens in Central Europe. 6. Babesia Ann Parasitol 64:265–284. https://doi.org/10.17420/ap6404.162
Kelly P, Koster L, Lobetti R (2015) Canine babesiosis: a perspective on clinical complications, biomarkers, and treatment. Vet Med Res Rep. https://doi.org/10.2147/vmrr.s60431
Vannier E, Krause PJ (2009) Update on babesiosis. Interdiscip Perspect Infect Dis 2009:1–9. https://doi.org/10.1155/2009/984568
Solano-Gallego L, Baneth G (2011) Babesiosis in dogs and cats-expanding parasitological and clinical spectra. Vet Parasitol 181:48–60. https://doi.org/10.1016/j.vetpar.2011.04.023
Solano-Gallego L, Sainz Á, Roura X, Estrada-Peña A, Miró G (2016) A review of canine babesiosis: the European perspective. Parasit Vectors 9:1–18. https://doi.org/10.1186/s13071-016-1596-0
Irwin PJ (2010) Canine babesiosis. Vet Clin North Am—Small Anim Pract 40:1141–1156. https://doi.org/10.1016/j.cvsm.2010.08.001
Mahalingaiah MKC, Asoor M, Thimmaiah RP, Narayanaswamy HD, Mukartal SY, Elattuvalappil AM, Chikkahonnaiah N, Gupta S, Singh S (2017) Prevalence of canine babesiosis in different breeds of dogs in and around Bengaluru. Adv Anim Vet Sci 5:140–144. https://doi.org/10.14737/journal.aavs/2017/5.3.140.144
Böse R, Jorgensen WK, Dalgliesh RJ, Friedhoff KT, de Vos AJ (1995) Current state and future trends in the diagnosis of babesiosis. Vet Parasitol 57:61–74. https://doi.org/10.1016/0304-4017(94)03111-9
Homer MJ, Aguilar-delfin I, Telford SR, Krause PJ, Persing DH (2000) Babesiosis. Clin Microb Rev 13:451–469
Aktaş M, Altay K, Dumanli N (2005) Development of a polymerase chain reaction method for diagnosis of Babesia ovis infection in sheep and goats. Vet Parasitol 133:277–281. https://doi.org/10.1016/j.vetpar.2005.05.057
Liu HH, Cushinotto L, Giger O, Daum G, McBride P, Negron EA, Vandegrift K, Kapelusznik L (2019) Increasing babesiosis in Southeastern Pennsylvania, 2008–2017. Open Forum Infect Dis 6:2008–2010. https://doi.org/10.1093/ofid/ofz066
Hartelt K, Rieker T, Oehme RM, Brockmann SO, Müller W, Dorn N (2007) First evidence of Babesia gibsoni (Asian genotype) in dogs in Western Europe. Vector-Borne Zoonotic Dis 7:163–166. https://doi.org/10.1089/vbz.2006.0580
Weilgama DJ, Jorgensen WK, Dalgliesh RJ, Navaratne M, Weerasinghe C (1989) Comparison between Sri Lankan and Australian strains of Babesia bovis in the vaccination of imported cattle in Sri Lanka. Trop Anim Health Prod 21:141–145. https://doi.org/10.1007/BF02236195
Neelawala D, Dissanayake DRA, Prasada DVP, Silva ID (2021) Analysis of risk factors associated with recurrence of canine babesiosis caused by Babesia gibsoni. Comp Immunol Microb Infect Dis 74:101572. https://doi.org/10.1016/j.cimid.2020.101572
Seneviratna P (1953) Piroplasmosis of dogs in Ceylon. Ceylon Veterinary J 1:95–98
Seneviratna P (1965) Studies of Babesia gibsoni infections of dogs in Ceylon. Ceylon Vet J 13:107–110
Seneviratna P (1965) Studies of Babesia gibsoni (patton, 1910) infections in the Dog. Br Vet J 121:263–271. https://doi.org/10.1016/s0007-1935(17)41153-5
Seneviratna P, Jayawwickrama SD (1961) Treatment of Babesia gibsoni infections in dogs with spirotrypan hoeschst. Indian Vet J 38:465–474
Jayathilake PS, Rajakaruna RS, Fernando ADS, Wijerathna HSU, Ginarathne KMH, Naullage NGRK, Silva SNS, Thananjayan K, Amarasiri LKHRT, Jayasundara NPK, Mallawa MCK, Dangolla A (2020) Canine vector-borne diseases of working military dogs of Sri Lanka air force, free-roaming and owned dogs. 10th Research Congress of the Postgraduate Institute of Science, RESCON. University of Peradeniya, p 140
Weerathunga D, Iddawela D (2019) Prevalence of canine tick-borne haemoparasites in three divisional secretariat divisions (Rambewa, Tirappane, and Galenbidunuwewa) in the Anuradhapura district, Sri Lanka. Sri Lanka J Infect Dis 9:111–119
Aragão H, Fonseca D (1961) Ixodological notesVII list and key to the representatives of the Brazilian ixodological fauna [In Portuguese]. Mem Inst Oswaldo Cruz 59:115–129
Labruna MB, Pereira MC (2001) Carrapato em cães no Brasil [In Portuguese]. Clínica Veterinária 30:24–32
Dantas-Torres F, Figueredo LA, Faustino MDG (2004) Ectoparasitos de cães provenientes de alguns municípios da região metropolitana do Recife, Pernambuco, Brasil [In Portuguese]. Rev Bras Parasitol Vet 13:151–154
Walker AR, Bouattour A, Camicas JL, Estrada- Peña A, Horak IG, Latif AA (2007) Ticks of domestic animals in Africa : a guide to identification of species. Bioscience Reports, Edinburgh Scotland, UK, pp 153–161
Casati S, Sager H, Gern L, Piffaretti JC (2006) Presence of potentially pathogenic Babesia sp. for human in Ixodes ricinus in Switzerland. Ann Agric Env Med 13:65–70
Kumar S, Stecher G, Tamura K (2016) MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33:1870–1874
Jain KJ, Lakshmanan B, Syamala K, Praveena JE, Aravindakshan T (2017) High prevalence of small Babesia species in canines of Kerala, South India. Vet World 10:1319–1323. https://doi.org/10.14202/vetworld.2017.1319-1323
Bhattacharjee K, Sarmah PC (2013) Prevalence of haemoparasites in pet, working and stray dogs of Assam and North-East India: a hospital based study. Vet World 6:874–878. https://doi.org/10.14202/vetworld.2013.874-878
Razi Jalali MH, Mosallanejad B, Avizeh R, Alborzil AR et al (2013) Babesia infection in urban and rural dogs in Ahvaz district, Southwest of Iran. Archiv Razi Inst 68:37–42
Dantas-Torres F, Otranto D (2014) Dogs, cats, parasites, and humans in Brazil: opening the black box. Parasit Vectors 7:1–25. https://doi.org/10.1186/1756-3305-7-22
Harrus S, Waner T, Bark H, Jongejan F, Cornelissen AWCA (1999) Recent advances in determining the pathogenesis of canine monocytic ehrlichiosis. J Clin Microb 37:2745–2749. https://doi.org/10.1128/jcm.37.9.2745-2749.1999
Zahler M, Rinder H, Schein E, Gothe R (2000) Detection of a new pathogenic Babesia microti-like species in dogs. Vet Parasitol 89:241–248. https://doi.org/10.1016/S0304-4017(00)00202-8
Macintire DK, Boudreaux MK, West GD, Bourne C, Wright JC, Conrad PA (2002) Babesia gibsoni infection among dogs in the South Eastern United States. J Am Vet Med Assoc 220:325–329. https://doi.org/10.2460/javma.2002.220.325
Silva I (2017) Complex clinical presentations of tick-borne diseases in dogs in Sri Lanka. Sri Lanka Vet J 63:1. https://doi.org/10.4038/slvj.v63i2.9
Bell AS, Ranford-Cartwright LC (2002) Real-time quantitative PCR in parasitology. Trends Parasitol 18:337–342. https://doi.org/10.1016/s1471-4922(02)02331-0
Sasaki M, Omobowale O, Tozuka M, Ohta K, Matsuu A, Nottidge HO, Hirata H, Ikadai H, Oyamada T (2007) Molecular survey of Babesia canis in dogs in Nigeria. J Vet Med Sci 69:1191–1193. https://doi.org/10.1292/jvms.69.1191
Acknowledgements
Authors wish to acknowledge the academic and non-academic staff at the VTH, at the Faculty of Veterinary Medicine and Animal Science, University of Peradeniya.
Funding
This work was supported by the National Science Foundation [grant number. RG/2019/BT/01] and the National Research Council [Grant no. 20-083], Sri Lanka.
Author information
Authors and Affiliations
Contributions
RASR: Sample collection and analysis, data analysis and writing of the manuscript. AD: sample collection, editing the manuscript; SDDSS: supervision of lab work and editing the manuscript; RSR: study conception and design, supervision, editing the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interests.
Ethical approval
The study protocols received ethical approval from the Ethical Review Committee of the Postgraduate Institute of Science (PGIS), University of Peradeniya, Sri Lanka.
Consent to participate
Verbal informed consent was obtained from the dog owners.
Consent for publication
Verbal informed consent was obtained from the dog owners.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ranatunga, R.A.S., Dangolla, A., Sooriyapathirana, S.D.S.S. et al. High Asymptomatic Cases of Babesiosis in Dogs and Comparison of Diagnostic Performance of Conventional PCR vs Blood Smears. Acta Parasit. 67, 1217–1223 (2022). https://doi.org/10.1007/s11686-022-00549-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11686-022-00549-x