Abstract
Purpose
Echinorhynchus gadi is one of the most widely distributed and commonly described acanthocephalans in marine fishes throughout the world. We provide a detailed morphometric and molecular description of a distinct Alaska population collected from the Pacific halibut Hippoglossus stenolepis Schmidt (Pleuronectidae) compared to those from other hosts and regions, illustrating new features never previously reported.
Methods
We described new specimens by microscopical studies, augmented by SEM, Energy Dispersive x-ray and molecular analyses, and histopathology.
Results
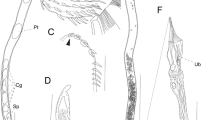
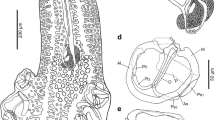
Specimens from Alaska were distinguished from those collected from the other geographical areas in proboscis size and its armature, especially number of hook rows and hooks per row, and length of hooks. The size of the receptacle, lemnisci, and reproductive structures in some other collections also varied from the Alaska material. X-ray scans of the gallium cut hooks depict prominent layering with high Sulfur content for tip cuts and increased calcium and phosphorus content in the base area of the hook. Sections of E. gadi specimens in the host tissue show prominent hook entanglement with subsequent connective tissue invasion also depicting the internal anatomy of certain worm structures not readily seen by other means. Molecular analyses clearly confirmed the identity of our E. gadi sequences.
Conclusion
Our Alaska population of the E. gadi complex appears to represent a novel population distinguishable by its distinct morphometrics, geography and host species. We further establish new information on the Energy Dispersive X-ray analysis in our Alaska material for future comparisons with the other siblings and explore genetic relationships among echinorhynchid genera and species.








Similar content being viewed by others
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Lühe M (1911) Acanthocephalen. In: Brauer A (ed) Die Süsswasserfauna Deutschlands. Verlag von Gustav Fischer, Jena, Germany, p 60
Meyer A (1932) Dr. H. G. Bronns Klassen und Ordnungen des Tierreichs. 4. Band, 2. Abt., 1. Buch Acanthocephala. Akademische Verlagsgesellschaft, Leipzig, Germany
Meyer A (1933) Dr. H. G. Bronns Klassen und Ordnungen des Tierreichs. 4. Band, 2. Abt., 2. Buch. Akademische Verlagsgesellschaft, Leipzig, Germany
Yamaguti S (1963) Systema Helminthum, vol V. Interscience Publishers, New York, USA, Acanthocephala
Golvan Y (1969) Systématique des Acanthocephales (Acanthocéphala Rudolphi, 1801), L’ordre des Palaeacanthocephala Meyer, 1931, La superfamille des Echinorhynchidea (Cobbold, 1876) Golvan et Houin 1973. Mémoires du Muséum Natl d’histoire Nat 47:1–373
Arai H (1989) Acanthocephala. In: Margolis L, Kabata Z (eds) Guide to the parasites of fishes of Canada. Part III, Otawa, pp 1–90
Van Cleave H (1924) A critical study of the acanthocephala described and identified by Joseph Leidy on JSTOR. Proc Acad Nat Sci Philadelphia 76:279–334
Amin OM (2013) Classification of the Acanthocephala. Folia Parasitol (Praha) 60:273–305. https://doi.org/10.14411/fp.2013.031
Petrochenko V (1956) Acanthocephala of domestic and wild animals, vol I. Izdatel’stvo Akademii Nauk SSSR, Moscow
Shostak AW, Dick TA, Szalai AJ, Bernier LMJ (1986) Morphological variability in Echinorhynchus gadi, E. leidyi, and E. salmonis (Acanthocephala: Echinorhynchidae) from fishes in northern Canadian waters. Can J Zool 64:985–995. https://doi.org/10.1139/z86-148
Miller R (1977) The biology of two species of Echinorhynchus (Acanthocephala) from marine fishes in Oregon. Ph.D. Thesis, Oregon State University, 109 pp.
Sobecka E, Szostakowska B, MacKenzie K, Hemmingsen W, Prajsnar S, Eydal M (2012) Genetic and morphological variation in Echinorhynchus gadi Zoega in Müller, 1776 (Acanthocephala: Echinorhynchidae) from Atlantic cod Gadus morhua L. J Helminthol 86:16–25. https://doi.org/10.1017/S0022149X10000891
Väinölä R, Valtonen E, Gibson DI (1994) Molecular systematics in the acanthocephalan genus Echinorhynchus (sensu lato) in northern Europe. Parasitology 108:105–114. https://doi.org/10.1017/S0031182000078574
Wayland MT, Gibson DI, Sommerville C (2005) Morphometric discrimination of two allozymically diagnosed sibling species of the Echinorhynchus gadi Zoega in Muller complex (Acanthocephala) in the North Sea. Syst Parasitol 60:139–149. https://doi.org/10.1007/s11230-004-1388-3
Wayland MT, Vainio JK, Gibson DI, Herniou EA, Littlewood DTJ, Väinölä R (2015) The systematics of Echinorhynchus Zoega in Müller, 1776 (Acanthocephala, Echinorhynchidae) elucidated by nuclear and mitochondrial sequence data from eight European taxa. Zookeys 484:25–52. https://doi.org/10.3897/zookeys.484.9132
Lee R (1992) Scanning electron microscopy and x-ray microanalysis. Prentice Hall, Englewood Cliffs, New Jersey, USA
Bancroft J, Gamble M (2001) Bancroft’s theory and practice of histological techniques, 5th edn. Churchill Livingston, Edenborough, U.K.
Kiernan J (2002) Histological and histochemical methods. Theory and Practice, Churchill Livingston, Edinburgh, U.K.
Galigher A, Kozloff E (1971) Essentials of practical microtechnique, 2nd edn. Lee and Febiger, Philadelphia, Pennsylvania, USA
Amin OM, Heckmann RA, Dallarés S, Constenla M, Van HN (2019) Morphological and molecular description of Rhadinorhynchus laterospinosus Amin, Heckmann & Ha, 2011 (Acanthocephala, Rhadinorhynchidae) from marine fish off the Pacific coast of Vietnam. Parasite 26:14. https://doi.org/10.1051/parasite/2019015
Fyler CA, Caira JN, Jensen K (2009) Five new species of Acanthobothrium (Cestoda: Tetraphyllidea) from an unusual species of Himantura (Rajiformes: Dasyatidae) from northern Australia. Folia Parasitol (Praha) 56:107–128. https://doi.org/10.14411/fp.2009.016
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30:2725–2729. https://doi.org/10.1093/molbev/mst197
Dereeper A, Guignon V, Blanc G, Audic S, Buffet S, Chevenet F, Dufayard JF, Guindon S, Lefort V, Lescot M, Claverie JM, Gascuel O (2008) Phylogeny.fr: robust phylogenetic analysis for the non-specialist. Nucleic Acids Res 36:W465–W469. https://doi.org/10.1093/nar/gkn180
Darriba D, Taboada GL, Doallo R, Posada D (2012) JModelTest 2: more models, new heuristics and parallel computing. Nat Methods 9:772
Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, Gascuel O (2010) New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol 59:307–321. https://doi.org/10.1093/sysbio/syq010
Ronquist F, Teslenko M, Van Der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP (2012) Mrbayes 3.2: efficient bayesian phylogenetic inference and model choice across a large model space. Syst Biol 61:539–542. https://doi.org/10.1093/sysbio/sys029
Miller MA, Pfeiffer W, Schwartz T (2010) Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In: 2010 Gateway Computing Environments Workshop, GCE 2010 New Orleans, Louisiana pp 1–8
Huelsenbeck JP, Ronquist F, Nielsen R, Bollback JP (2001) Bayesian inference of phylogeny and its impact on evolutionary biology. Science 294(5550):2310–2314. https://doi.org/10.1126/science.1065889
Blaylock RB, Holmes JC, Margolis L (1998) The parasites of Pacific halibut (Hippoglossus stenolepis) in the eastern North Pacific: host-level influences. Can J Zool 76:536–547. https://doi.org/10.1139/z97-214
Müller O (1776) Zoologiae danicae prodromus seu animalium daniae et norvegiae indigenarum characters, nomina, et synonyma imprimis popularium. Havniae XXXII, Havniae: typis Hallageriis
Verweyen L, Klimpel S, Palm HW (2011) Molecular phylogeny of the Acanthocephala (class Palaeacanthocephala) with a paraphyletic assemblage of the orders polymorphida and echinorhynchida. PLoS ONE 6:28285. https://doi.org/10.1371/journal.pone.0028285
Amin OM, Heckmann RA (2017) Neoandracantha peruensisn. gen. n. sp. (Acanthocephala, Polymorphidae) described from cystacanths infecting the ghost crab Ocypode gaudichaudii on the Peruvian coast. Parasite 24:40
Heckmann RA, Amin OM, Radwan NA, Standing MD, Eggett DL, El Naggar AM (2012) Fine structure and energy dispersive x-ray analysis (EDXA) of the proboscis hooks of Rhadinorhynchus ornatus, Van Cleave 1918 (Rhadinorhynchidae: Acanthocephala). Sci Parasitol 13:37–43
Standing M, Heckmann R (2014) Features of Acanthocephalan hooks using dual beam preparation and XEDS phase maps. Microscopy and microanalysis meeting. Hartford, Connecticut, U.S.A., pp 0383–00501
Amin OM, Heckmann RA, Zargar UR (2017) Description of a new quadrigyrid Acanthocephalan from Kashmir, with notes on metal analysis and histopathology, and a key to species of the subgenus Acanthosentis from the Indian Subcontinent. J Parasitol 103:458–470. https://doi.org/10.1645/17-27
Van HN, Amin OM, Ngo HD, Heckmann RA (2018) Descriptions of acanthocephalans, Cathayacanthus spinitruncatus (Rhadinorhynchidae) male and Pararhadinorhynchus magnus n. sp. (Diplosentidae), from marine fish of Vietnam, with notes on Heterosentis holospinus (Arhythmacanthidae). Parasite 25:35. https://doi.org/10.1051/parasite/2018032
Amin OM, Heckmann RA, Van Ha N (2018) Descriptions of Neorhadinorhynchus nudum (Cavisomidae) and Heterosentis paraholospinus n. sp. (Arhythmacanthidae) (Acanthocephala) from fish along the Pacific Coast of Vietnam, with notes on biogeography. J Parasitol 104:486–495. https://doi.org/10.1645/17-176
Amin OM, Heckmann RA, Sharifdini M, Albayati NY (2019) Moniliformis cryptosaudi n. sp. (Acanthocephala: Moniliformidae) from the long-eared hedgehog Hemiechinus auritus (Gmelin) (Erinaceidae) in Iraq; a case of incipient cryptic speciation related to M. saudi in Saudi Arabia. Acta Parasitol 64:195–204. https://doi.org/10.2478/s11686-018-00021-9
Giribet G, Sørensen MV, Funch P, Kristensen RM, Sterrer W (2004) Investigations into the phylogenetic position of Micrognathozoa using four molecular loci. Cladistics 20:1–13. https://doi.org/10.1111/j.1096-0031.2004.00004.x
Malyarchuk B, Derenko M, Mikhailova E, Denisova G (2014) Phylogenetic relationships among Neoechinorhynchus species (Acanthocephala: Neoechinorhynchidae) from North-East Asia based on molecular data. Parasitol Int 63:100–107. https://doi.org/10.1016/j.parint.2013.09.012
Tkach VV, Lisitsyna OI, Crossley JL, Binh TT, Bush SE (2013) Morphological and molecular differentiation of two new species of Pseudoacanthocephalus Petrochenko, 1958 (Acanthocephala: Echinorhynchidae) from amphibians and reptiles in the Philippines, with identification key for the genus. Syst Parasitol 85:11–26. https://doi.org/10.1007/s11230-013-9409-8
Nakao M (2016) Pseudoacanthocephalus toshimai sp. nov. (Palaeacanthocephala: Echinorhynchidae), a common acanthocephalan of anuran and urodelan amphibians in Hokkaido, Japan, with a finding of its intermediate host. Parasitol Int 65:323–332. https://doi.org/10.1016/j.parint.2016.03.011
Amin OM, Heckmann RA, Fiser Z, Zaksek V, Herlyn H, Kostanjsek R (2019) Description of Acanthocephalus anguillae balkanicus subsp. n. (Acanthocephala: Echinorhynchidae) from Proteus anguinus Laurenti (Amphibia: Proteidae) and the cave ecomorph of Asellus aquaticus (Crustacea: Asellidae) in Slovenia. Folia Parasitol (Praha) 66:2019015. https://doi.org/10.14411/fp.2019.015
Acknowledgements
We thank Madison Laurence, Bean Museum (BYU) for expert help in the preparation and organization of plates and figures and Michael Standing, Electron Optics Laboratory (BYU), for his technical help and expertise in the generation of SEM images and EDXA profiles.
Funding
This project was supported by the Department of Biology, Brigham Young University (BYU), Provo, Utah and by an Institutional Grant from the Parasitology Center, Inc. (PCI), Scottsdale, Arizona.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standards
Fieldwork was run according to acceptable relevant ethical standards.
Consent for publication
All authors have agreed to be listed and approved the final version of the manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Amin, O.M., Heckmann, R.A., Dallarés, S. et al. Morphological and molecular description of a distinct population of Echinorhynchus gadi Zoega in Müller, 1776 (Paleacanthocephala: Echinorhynchidae) from the pacific halibut Hippoglossus stenolepis Schmidt in Alaska. Acta Parasit. 66, 881–898 (2021). https://doi.org/10.1007/s11686-021-00361-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11686-021-00361-z