Abstract
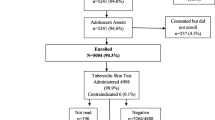
The prevalence of tuberculosis infection among adolescents born after terminating the Bacillus Calmette–Guérin (BCG) booster vaccination in China was estimated using tuberculin skin testing (TST) and QuantiFERON-TB Gold assay (QFT) to investigate the influence of neonatal BCG vaccination on the performance of TST. Data analysis was conducted for 2831 eligible participants aged 5–15 years from the baseline survey of a population-based multi-center prospective study. The prevalence rates of TST (induration = 10 mm) and QFT positivity were 9.3% (264/2827) and 2.5% (71/2831), respectively. The rate of QFT indeterminate result was 2.2% (62/2831). The overall agreement between TST and QFT was low (concordance = 88.0%; ? coefficient = 0.125). Only TST was positively associated with BCG vaccination with an adjusted odds ratio of 1.71 [95% confidence interval, 1.26–2.31]. A history of close contact with patients of active TB was significantly associated with positivity for TST and QFT. Our results suggested that BCG neonatal vaccination still affects TST performance, and a twostep approach might be considered for TB infection testing among adolescents in China.
Similar content being viewed by others
References
Huebner RE, Schein MF, Bass JB Jr. The tuberculin skin test. Clin Infect Dis 1993; 17(6): 968–975
Menzies D. What does tuberculin reactivity after bacille Calmette-Guérin vaccination tell us? Clin Infect Dis 2000; 31(Suppl 3): S71–S74
Pai M, Riley LW, Colford JM Jr. Interferon-γ assays in the immunodiagnosis of tuberculosis: a systematic review. Lancet Infect Dis 2004; 4(12): 761–776
Andersen P, Munk ME, Pollock JM, Doherty TM. Specific immunebased diagnosis of tuberculosis. Lancet 2000; 356(9235): 1099–1104
Wang L, Liu J, Chin DP. Progress in tuberculosis control and the evolving public-health system in China. Lancet 2007; 369(9562): 691–696
Gao L, Lu W, Bai L, Wang X, Xu J, Catanzaro A, Cárdenas V, Li X, Yang Y, Du J, Sui H, Xia Y, Li M, Feng B, Li Z, Xin H, Zhao R, Liu J, Pan S, Shen F, He J, Yang S, Si H, Wang Y, Xu Z, Tan Y, Chen T, Xu W, Peng H, Wang Z, Zhu T, Zhou F, Liu H, Zhao Y, Cheng S, Jin Q; LATENTTB-NSTM study team. Latent tuberculosis infection in rural China: baseline results of a population-based, multicentre, prospective cohort study. Lancet Infect Dis 2015; 15(3): 310–319
Gao L, Jin Q. Differences in BCG vaccination and tuberculin skintest positivity—Authors’ reply. Lancet Infect Dis 2015; 15(9): 1003–1005
Mazurek GH, Jereb J, Lobue P, Iademarco MF, Metchock B, Vernon A, Division of Tuberculosis Elimination, National Center for HIV, STD, and TB Prevention, Centers for Disease Control and Prevention (CDC). Guidelines for using the QuantiFERON-TB Gold test for detecting Mycobacterium tuberculosis infection, United States. MMWR Recomm Rep 2005; 54(RR-15): 49–55
Cheng S, Wang G, Wang L, Zhou L. Guidance of Tuberculin Skin Test. Bengjing: People’s Medical Publishing House, 2014
Sheng YL. China Statistical Yearbook—2010. Beijing: China Statistics Press, 2010
World Health Organization. Growth reference 5–19 years. 2007. http://www.who.int/growthref/who2007_bmi_for_age/en/ (Accessed October 8, 2016)
Kang YA, Lee HW, Yoon HI, Cho B, Han SK, Shim YS, Yim JJ. Discrepancy between the tuberculin skin test and the whole-blood interferon γ assay for the diagnosis of latent tuberculosis infection in an intermediate tuberculosis-burden country. JAMA 2005; 293(22): 2756–2761
Mandalakas AM, Detjen AK, Hesseling AC, Benedetti A, Menzies D. Interferon-γ release assays and childhood tuberculosis: systematic review and meta-analysis. Int J Tuberc Lung Dis 2011; 15(8): 1018–1032
Machingaidze S, Wiysonge CS, Gonzalez-Angulo Y, Hatherill M, Moyo S, Hanekom W, Mahomed H. The utility of an interferon γ release assay for diagnosis of latent tuberculosis infection and disease in children: a systematic review and meta-analysis. Pediatr Infect Dis J 2011; 30(8): 694–700
World Health Organization. Use of tuberculosis interferon-γ release assays (IGRAs) in low-and middle-income countries: policy statement. 2011. http://whqlibdoc.who.int/publications/2011/9789241502672_eng (Accessed August 9, 2016)
Mazurek GH, Jereb J, Vernon A, LoBue P, Goldberg S, Castro K; IGRA Expert Committee; Centers for Disease Control and Prevention (CDC). Updated guidelines for using interferon γ release assays to detect Mycobacterium tuberculosis infection — United States, 2010. MMWR Recomm Rep 2010; 59(RR-5): 1–25
National Institute for Health and Care Excellence. Clinical diagnosis and management of tuberculosis, and measures for its prevention and control. Manchester: NICE, 2011
Lighter J, Rigaud M, Eduardo R, Peng CH, Pollack H. Latent tuberculosis diagnosis in children by using the QuantiFERON-TB Gold In-Tube test. Pediatrics 2009; 123(1): 30–37
Tsiouris SJ, Austin J, Toro P, Coetzee D, Weyer K, Stein Z, El-Sadr WM. Results of a tuberculosis-specific IFN-γ assay in children at high risk for tuberculosis infection. Int J Tuberc Lung Dis 2006; 10 (8): 939–941
Bianchi L, Galli L, Moriondo M, Veneruso G, Becciolini L, Azzari C, Chiappini E, de Martino M. Interferon-γ release assay improves the diagnosis of tuberculosis in children. Pediatr Infect Dis J 2009; 28(6): 510–514
Nelson LJ, Wells CD. Global epidemiology of childhood tuberculosis. Int J Tuberc Lung Dis 2004; 8(5): 636–647
Starke JR. Childhood tuberculosis: ending the neglect. Int J Tuberc Lung Dis 2002; 6(5): 373–374
Piñeiro R, Mellado MJ, Cilleruelo MJ, García-Ascaso M, Medina-Claros A, García-Hortelano M. Tuberculin skin test in bacille Calmette-Guérin-vaccinated children: how should we interpret the results? Eur J Pediatr 2012; 171(11): 1625–1632
Rieder HL. Epidemiological Basis of TB Control. Paris: International Union Against Tuberculosis and Lung Disease, 1999. 26–43
Wang L, Turner MO, Elwood RK, Schulzer M, FitzGerald JM. A meta-analysis of the effect of Bacille Calmette Guérin vaccination on tuberculin skin test measurements. Thorax 2002; 57(9): 804–809
Farhat M, Greenaway C, Pai M, Menzies D. False-positive tuberculin skin tests: what is the absolute effect of BCG and nontuberculous mycobacteria? Int J Tuberc Lung Dis 2006; 10(11): 1192–1204
Mahomed H, Hawkridge T, Verver S, Geiter L, Hatherill M, Abrahams DA, Ehrlich R, Hanekom WA, Hussey GD; SATVI Adolescent Study Team. Predictive factors for latent tuberculosis infection among adolescents in a high-burden area in South Africa. Int J Tuberc Lung Dis 2011; 15(3): 331–336
Connell TG, Ritz N, Paxton GA, Buttery JP, Curtis N, Ranganathan SC. A three-way comparison of tuberculin skin testing, Quanti-FERON-TB gold and T-SPOT.TB in children. PLoS One 2008; 3 (7): e2624
Gao L, Bai L, Liu J, Lu W, Wang X, Li X, Du J, Chen X, Zhang H, Xin H, Sui H, Li H, Su H, He J, Pan S, Peng H, Xu Z, Catanzaro A, Evans TG, Zhang Z, Ma Y, Li M, Feng B, Li Z, Guan L, Shen F, Wang Z, Zhu T, Yang S, Si H, Wang Y, Tan Y, Chen T, Chen C, Xia Y, Cheng S, Xu W, Jin Q; LATENTTB-NSTM study team. Annual risk of tuberculosis infection in rural China: a population-based prospective study. Eur Respir J 2016; 48(1): 168–178
Joshi M, Monson TP, Joshi A, Woods GL. IFN-γ release assay conversions and reversions. Challenges with serial testing in U.S. health care workers. Ann Am Thorac Soc 2014; 11(3): 296–302
Slater ML, Welland G, Pai M, Parsonnet J, Banaei N. Challenges with QuantiFERON-TB Gold assay for large-scale, routine screening of U.S. healthcare workers. Am J Respir Crit Care Med 2013; 188(8): 1005–1010
Machado A Jr, Emodi K, Takenami I, Finkmoore BC, Barbosa T, Carvalho J, Cavalcanti L, Santos G, Tavares M, Mota M, Barreto F, Reis MG, Arruda S, Riley LW. Analysis of discordance between the tuberculin skin test and the interferon-γrelease assay. Int J Tuberc Lung Dis 2009; 13(4): 446–453
Kobashi Y, Sugiu T, Mouri K, Obase Y, Miyashita N, Oka M. Indeterminate results of QuantiFERON TB-2G test performed in routine clinical practice. Eur Respir J 2009; 33(4): 812–815
Lange B, Vavra M, Kern WV, Wagner D. Indeterminate results of a tuberculosis-specific interferon-γ release assay in immunocompromised patients. Eur Respir J 2010; 35(5): 1179–1182
Zrinski Topic R, Zoricic-Letoja I, Pavic I, Dodig S. Indeterminate results of QuantiFERON-TB Gold In-Tube assay in nonimmunosuppressed children. Arch Med Res 2011; 42(2): 138–143
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Li, H., Xin, H., Qian, S. et al. Testing of tuberculosis infection among Chinese adolescents born after terminating the Bacillus Calmette–Guérin booster vaccination: subgroup analysis of a population-based cross-sectional study. Front. Med. 11, 528–535 (2017). https://doi.org/10.1007/s11684-017-0573-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11684-017-0573-0