Abstract
Conscious experience and perception are restricted to a single perspective. Although evidence to suggest differences in phenomenal experience can produce observable differences in behavior, it is not well understood how these differences might influence memory. We used fMRI to scan n = 49 participants while they encoded and performed a recognition memory test for faces and words. We calculated a cognitive bias score reflecting individual participants’ propensity toward either Visual Imagery or Internal Verbalization based on their responses to the Internal Representations Questionnaire (IRQ). Neither visual imagery nor internal verbalization scores were significantly correlated with memory performance. In the fMRI data, there were typical patterns of activation differences between words and faces during both encoding and retrieval. There was no effect of internal representation bias on fMRI activation during encoding. At retrieval, however, a bias toward visualization was positively correlated with memory-related activation for both words and faces in inferior occipital gyri. Further, there was a crossover interaction in a network of brain regions such that visualization bias was associated with greater activation for words and verbalization bias was associated with greater activation for faces, consistent with increased effort for non-preferred stimulus retrieval. These findings suggest that individual differences in cognitive representations affect neural activation across different types of stimuli, potentially affecting memory retrieval performance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Individuals differ in their phenomenal experience of the external world. This is also true for internal representations; some individuals expressing extremely vivid visual imagery (Cui et al., 2007; Marks, 1973) and others expressing an absence of visual imagery (i.e., aphantasia; Keogh & Pearson, 2018). Similarly, some individuals express strong internal verbalization (Alderson-Day et al., 2018) and others report an absence of this experience (Heavey & Hurlburt, 2008), which has recently been termed “anendophasia” (Nedergaard & Lupyan, 2023). Recent evidence suggests that the phenomenal experience may influence performance in various cognitive domains. Participants who self-report having lower levels of inner speech have been shown to have lower performance on verbal working memory and rhyming judgment tasks (Nedergaard & Lupyan, 2023). Further, aphantasic participants, who report no ability to visualize despite no neurological damage, have been found to have generally reduced performance for episodic memory tasks, specifically retrieving episodic detail, compared to control groups (Blomkvist, 2023; Milton et al., 2021). Although previous literature has examined the effects of phenomenal experience for visual imagery and internal verbalization on memory, it is unclear how these experiences interact when forming and retrieving memories for verbal or visual information.
Prior research on individual differences in other domains has demonstrated effects on memory encoding and retrieval processes. For example, stable, non-strategic individual differences in recognition memory ability that are persistent over short (e.g., one week; Cohen, 1984) and long (e.g., 1–4 years; Woodhead & Baddeley, 1981; Zerr et al., 2018) delays. Further, individual differences in personality (Megreya & Bindemann, 2013) and mindfulness (Giannou et al., 2020) have been associated with memory performance for faces and encoding strategy (Karis et al., 1984) has been associated with memory performance for words.
A recently-developed tool called the Internal Representations Questionnaire (IRQ) provides a measure of individual differences in phenomenal experience, namely the propensity to use one cognitive style over another (Roebuck & Lupyan, 2020). The authors identify four factors of internal representation: Visual Imagery, Internal Verbalization, Orthographic Imagery, and Representational Manipulation. IRQ sub-scale scores for Visual Imagery and Internal Verbalization correlated with performance at visual and language-based tasks, respectively (Roebuck & Lupyan, 2020). This new measure provides the opportunity to use a more empirical examination of cognitive style, for both visual and verbal preference using the same tool, to determine if it has an observable impact on memory test performance. Likewise, it also provides an avenue to examine whether corresponding differences in neural responses exist. We sought to answer whether individual differences as measured by the IRQ would influence memory recognition performance for visual and verbal stimuli, and if such differences would produce differential activation at encoding and retrieval. Specifically, we hypothesized that individuals who had more of a preference for internal verbalization would have better memory performance for verbal stimuli and those who had more of a preference for visual imagery would have better memory performance for visual stimuli (i.e., faces). Further, we hypothesized that brain regions associated with linguistic and visual processing would demonstrate differential activation corresponding to these behavioral effects.
Methods
Participants
A total of 120 participants were recruited from the university and nearby community. All participants self-reported free of neurological or psychiatric diagnoses and met safety inclusion criteria for MRI scanning (e.g., screening implanted medical devices). Participants were originally recruited for a study on the impacts of handedness on memory function (handedness analyses reported elsewhere). Consequently, 61 participants were excluded from the present analyses due left-handedness (Edinburgh Handedness Inventory Scores < 40). Further exclusions included seven participants due to excessive motion in the MRI scanner; two due to low response rates in the memory task (nonresponses > 2 Standard Deviations above the mean); and one due to being a notably strong outlier for cognitive bias score (-3.9 Standard Deviations below the mean). The final data analyses were performed with n = 49 (25 male; 24 female, mean age = 23.1, range 18 to 48).
Procedure
Prior to MRI scanning, participants completed the Internal Representations Questionnaire (IRQ), which is composed of four subscales: Visual Imagery, Internal Verbalization, Orthographic Imagery, and Representational Manipulation. We focused on the Visual Imagery and Internal Verbalization subscales given our research interest in whether the propensity to engage in visual imagery or internal verbalization affected memory for verbal or non-verbal stimuli.
While in the MRI scanner, participants performed encoding tasks for words then faces in separate blocks, a semantic fluency task (data used in a separate analysis; in preparation), and retrieval blocks for words then faces. We collected T1- and T2-weighted structural scans as well as a field map scan following the semantic fluency task prior to the retrieval task blocks, resulting in a delay between encoding and retrieval of approximately 15 min.
For the encoding task, participants rated as “pleasant” or “unpleasant” a series of 100 words and then 100 faces in separate scan runs (Guerin & Miller, 2009). Stimulus types were presented in separate runs to avoid set shifting effects that may impair subsequent memory performance (Muhmenthaler & Meier, 2019). Both face and word stimuli were presented for 2.5 s each followed by a fixation cross for 0.5–1.5 s, jittered (Amaro & Barker, 2006). Stimulus order was randomized for both encoding and retrieval. Face stimuli were selected from a database (Minear & Park, 2004) to have a broad distribution of demographics (e.g., age, sex, ethnicity). Word stimuli were selected from the MRC psycholinguistic database (Coltheart, 1981) to have high familiarity and high concreteness. For the retrieval task, participants performed recognition memory tasks with faces and words in separate scan runs again. Face and word recognition memory tests included 100 targets and 100 foils for each stimulus type. Within each block retrieval tasks were broken into two scan runs of 100 trials each, totaling 200 trials for words, and 200 trials for faces. Stimuli were presented one at a time for 2.5 s while participants made “old/new” recognition memory judgments. The inter-stimulus interval was again a fixation cross for 0.5–1.5 s, jittered. Responses were recorded using a four-button MR-compatible response cylinder (Current Designs Inc.; Philadelphia, PA). Stimuli were displayed using an MR-compatible LCD monitor placed at the head of the MRI scanner viewable using an adjustable mirror attached to the head coil.
MRI scanning
All MRI imaging was performed on a Siemens 3 Tesla TIM Trio scanner (Erlangen, Germany), using a 32-channel head coil. Each participant contributed a T1-weighted MP-RAGE structural scan (176 slices; TR = 1900 ms; TE = 4.92 ms; flip angle = 9°; field of view = 256 mm; slice thickness = 1 mm; voxel resolution = 0.97× 0.97 × 1.0 mm; 1 average) and echo-planar imaging (EPI) scans for each of the task blocks (72 interleaved slices; TR = 1800 ms; TE = 42 ms; flip angle 90°; field of view = 180 mm; slice thickness = 1.8 mm; voxel resolution = 1.8 × 1.8 × 1.8 mm; Multi-Band factor = 4).
Imaging data analysis
Unless otherwise noted, data were analyzed with AFNI (version AFNI_19.2.22) and SPSS (v.28). DICOM images were converted to NIfTI using dcm2niix (Li et al., 2016) and de-faced for anonymity. NifTI files were then uploaded to BrainLife.io (Avesani et al., 2019) and preprocessed using FreeSurfer and fMRIprep pipelines. Detailed descriptions of the fMRIprep pipeline autogenerated by the program are in Supplemental Materials. Additionally, functional data were blurred with a 4 mm FWHM Gaussian blur and scaled to have a mean value of 100 and range 0-200. Large motion events, defined as TRs with Euclidean norm (ENORM) of the temporal derivative of motion estimates greater than 0.3, were censored from the time series along with TRs immediately before and after.
Separate single subject regression models were created for face encoding, word encoding, face retrieval, and word retrieval tasks. Encoding task behavioral regressors coded for subsequent hits, subsequent misses, and trials of no interest including nonresponses. Retrieval task regressors coded for hits, misses, correct rejection (CRs), false alarms (FAs) and trials of no interest. For all tasks, events were modeled as a canonical hemodynamic response function convolved with a boxcar function of 2.5-second duration. All regression models included regressors for motion and polynomial regressors coding for run (retrieval tasks had two scan runs) and scanner drift. Single subject regression models did not include regressors for cognitive bias scores. Resulting statistical maps of fit coefficients (β-coefficients) were entered into group-level analyses. Group-level repeated-measures ANOVAs were conducted on memory-related activation using AFNI program 3dMVM with stimulus type (faces, words) as a within-subject factor and participant cognitive bias as a continuous between-subject factor. Separate ANOVAs were conducted for encoding and retrieval data. Memory-related activation at encoding was defined as subsequent hits minus subsequent misses. Similarly, memory-related activation at retrieval was defined as hits minus correct rejections. Whole-brain analyses were corrected for multiple comparisons by first performing Monte Carlo simulations where smoothness of the data was estimated from the residuals of the single-subject regression analyses (Cox et al., 2017). Family-wise error was set to p < .05 with a voxel-wise threshold of p < .01 (Mongelli et al., 2017; Monteleone et al., 2009) and a spatial extent threshold k > 88 for all analyses. To characterize the direction of the resultant group level activations, beta values from the single subject regressions were extracted from clusters defined by the group-level analyses using 3dROIstats. Relationships between beta values and respective cognitive bias scores were characterized using SPSS.
Behavioral data analysis
We calculated discriminability (d’) scores for faces and words separately as z(hits)-z(false alarms). Given our focus on the relationship between visual imagery, internal verbalization, and memory processes, we first z-transformed raw visual imagery and internal verbalization sub-scale scores from the IRQ. Previous research has demonstrated that visual imagery and internal verbalization IRQ scores are positively correlated (Roebuck & Lupyan, 2020). Accordingly, in order to identify the differential influence of verbalization and visualization, we created a cognitive bias score by taking the difference between visual imagery and internal verbalization z-scores, i.e., z(visual imagery) – z(internal verbalization). Thus, positive scores indicate a greater propensity toward visual imagery and negative scores indicate a propensity toward internal verbalization. SPSS was used to obtain bivariate Pearson Correlations for face d’ by cognitive bias and word d’ by cognitive bias.
Data and code availability
MRI data are available at https://openneuro.org/datasets/ds004589. Code used to present stimuli and perform all analyses is available at https://osf.io/tr78u/.
Results
Behavioral results
Overall memory performance was significantly above chance for faces (mean d’ = 1.5; SD = 0.4; t[48] = 26.40, p < .001, 95%CI [1.40, 1.63]) and for words (mean = 2.9; SD = 0.7; t[48] = 28.09, p < .001, 95%CI [2.70, 3.11]). The correlation coefficient for face memory performance and cognitive bias score was positive but failed to reach statistical significance (r = .081, p = .582), while the correlation coefficient for word memory performance was negative but also failed to reach statistical significance (r = − .206, p = .156). Further, the difference in correlations failed to reach significance (z=-1.547, p = .061) (Fig. 1).
Memory Performance by Cognitive Bias Score. Scatter plots with regression line displaying discriminability (d’) scores for faces (A) and words (B) against individual cognitive bias scores. Positive scores indicate bias toward visual imagery. Cognitive bias did not significantly affect face memory (r = .081, p = .582) or word memory (r = − .206, p = .156)
Imaging results
We performed separate analyses for encoding and retrieval tasks by performing whole-brain voxel-wise repeated-measures ANOVAs on memory-related activation with stimulus (words, faces) as a within-subject factor, and cognitive bias score as a continuous between-subjects factor. At both encoding and retrieval, there was widespread activation for the main effect of stimulus type, consistent with previous research demonstrating differential responding to faces compared to words (see Supplementary Materials for activation maps). At encoding, there were no significant clusters for the main effect of cognitive bias or for the stimulus by cognitive bias interaction. At retrieval, there were two clusters of activation in bilateral inferior occipital lobe (left: 213 voxels, MNI coordinates − 41, -91, -8; right: 97 voxels, MNI coordinates 33, -97, -7) that demonstrated a significant main effect of cognitive bias. The left cluster encompassed parts of the V2, V3, and V4 visual area, as well as parts of the posterior inferotemporal gyrus. The right cluster included V2, V3, and V4 visual areas. In both clusters there was a significant positive relationship between cognitive bias and activation, such that a cognitive bias toward visualization was associated with greater retrieval activation, collapsing across faces and words (Fig. 2).
Main effect of cognitive bias at retrieval in left (A) and right (C) inferior occipital gyrus (B). Memory retrieval related fMRI activation was defined as Hits-CRs (collapsed across faces and words). A positive cognitive bias score reflects an individual’s propensity toward visual imagery. In both left and right inferior occipital gyrus there was a positive relationship between visual imagery bias and memory retrieval related fMRI activation
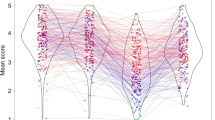
Additionally, there were five clusters of activation at retrieval that demonstrated significant crossover interactions for cognitive bias by stimulus type (Table 1). These clusters included left lateral middle temporal gyrus, left inferior frontal gyrus orbitalis, left middle orbital gyrus, right inferior frontal gyrus orbitalis, and right angular gyrus (Fig. 3A). These clusters all demonstrated a similar pattern of activity where a greater bias toward internal verbalization was associated with greater memory-related activation for faces, and a greater bias toward visual imagery was associated with greater memory-related activation for words (Fig. 3B).
Clusters demonstrating a cognitive bias by stimulus type interaction at retrieval (A). All clusters followed a similar pattern of activity, illustrated by the activation from the largest cluster in left lateral middle temporal gyrus, in which visual imagery bias was associated with greater activation for words (B), and less activation for faces. Conversely, internal verbalization bias was associated with the opposite pattern (C)
Discussion
The primary aims of this study were twofold. First, we asked whether phenomenal experience as measured by the cognitive bias score would have a relationship with memory performance. Second, we asked whether the cognitive bias score would be associated with differential activation at encoding or retrieval for verbal and non-verbal stimuli. Our results for the former were not significant, but this may be due to low power. For the latter question, we did not observe any effects of phenomenal experience on fMRI activation for memory encoding regardless of stimulus type. However, during memory retrieval we found a main effect of cognitive bias for two clusters of activation in early visual cortex. Within these clusters higher visual bias was associated with greater memory retrieval related activation for both words and faces. Additionally, we also observed a network of brain regions that displayed a cross-over interaction of cognitive bias by stimulus type, discussed below.
Behavioral findings
Our research question stems from previous findings demonstrating relationships between individual differences in non-mnemonic cognition and memory performance. Here, we asked if individual differences corresponding to phenomenal experience for verbal and visual information were associated with recognition memory performance for verbal and visual stimuli. Our findings did not demonstrate a relationship between cognitive bias score and memory performance. This is in contrast to previous studies finding significant effects of individual differences on memory performance (Nedergaard & Lupyan, 2023; Woodhead & Baddeley, 1981). We suggest that the current study may not be sufficiently powered to address the question of whether cognitive style affects memory performance (Anderson et al., 2017).
Neural findings
We observed greater activation (Hits-CRs) during memory retrieval in the inferior occipital gyri for visual biased participants collapsed across faces and words. This result may indicate that a propensity toward visual imagery produces greater recruitment of visual regions for memory retrieval tasks in general. This is consistent with previous research that has demonstrated a connection between visual imagery and face perception in occipital activation (Ishai, 2002; Slotnick et al., 2012). We found activations particularly in early visual cortex, V2 and V3 activations occurred for both left and right hemispheres. Early visual cortex activations have also been associated with visual imagery tasks (Albers et al., 2013; Dijkstra et al., 2017; Pearson, 2019). Previous literature has demonstrated the ability to decode imagery content to some extent using V2 activation (Pearson, 2019). That these significant activations occurred during retrieval and not simultaneously at encoding suggests these differences are not due to differences in visual perception, but rather may be due to engaging in some level of imagery involved in memory retrieval. Greater activation in these areas for memory retrieval may suggest that individuals with a bias toward visual imagery are more likely to recruit early visual areas as they reconstruct a memory in a visual form regardless of the task format itself.
We also found a significant cognitive style by stimulus type cross-over interaction at retrieval reflecting greater memory activity when stimulus type did not match preferred cognitive bias. Active regions overlapped with the language network (left middle temporal gyrus and inferior frontal gyrus), the frontoparietal control network (inferior frontal and middle orbital gyrus), and the default mode network (right angular gyrus). A recent model of memory retrieval (Kim, 2020) suggests that the frontoparietal control network supports retrieval and decision effort while the medial temporal lobe (MTL) supports retrieval of memory representations and the DMN supports the subjective experience of remembering. While we did not observe differential activation of the MTL (consistent with activation across stimulus types and cognitive biases), we did observe differential activation consistent with this account in the FPCN and DMN. Also consistent with an interpretation of greater effort are studies that demonstrate increased activation in similar networks for linguistic task demands in (Klimovich-Gray et al., 2017).
We did not observe any effects of cognitive bias on memory encoding. Prior research on individual differences for mindfulness demonstrated effects at retrieval and not encoding, suggesting differences in decision-making strategy (Rosenstreich & Ruderman, 2016). Cognitive bias may similarly influence memory-guided decision-making strategy. Alternatively, the phenomenal experience of memory retrieval may vary according to cognitive bias, where the experience of autonoetic consciousness (Tulving, 2002) corresponds to preferred cognitive bias. Future research will be needed to characterize this possibility. Similarly, further research may wish to examine if internal imagery has an interaction with word concreteness for memory recognition (Taylor et al., 2019).
Conclusions
Our findings demonstrate an effect of phenomenal style on memory retrieval activation. Participants with a bias toward visual imagery differentially activated visual cortices during memory retrieval regardless of stimulus type. We observed a further pattern of activation consistent with task demands, as activation was greater when cognitive bias and task type differed. Taken together, our findings suggest that differences in phenomenal experience are reflected in neural activation in retrieval tasks. This variability in retrieval activation should be taken into account in our theorizing about how brain networks interact in support of episodic memory retrieval.
Data availability
MRI data are available at https://openneuro.org/datasets/ds004589. Code used to present stimuli and perform all analyses is available at https://osf.io/tr78u/.
References
Albers, A. M., Kok, P., Toni, I., Dijkerman, H. C., & de Lange, F. P. (2013). Shared representations for Working Memory and Mental Imagery in early visual cortex. Current Biology, 23(15), 1427–1431. https://doi.org/10.1016/j.cub.2013.05.065
Alderson-Day, B., Mitrenga, K., Wilkinson, S., McCarthy-Jones, S., & Fernyhough, C. (2018). The varieties of inner speech questionnaire – revised (VISQ-R): Replicating and refining links between inner speech and psychopathology. Consciousness and Cognition, 65, 48–58. https://doi.org/10.1016/j.concog.2018.07.001
Amaro, E., & Barker, G. J. (2006). Study design in fMRI: Basic principles. Brain and Cognition, 60(3), 220–232. https://doi.org/10.1016/j.bandc.2005.11.009
Anderson, S. F., Kelley, K., & Maxwell, S. E. (2017). Sample-size planning for more Accurate Statistical Power: A Method Adjusting Sample Effect sizes for Publication Bias and uncertainty. Psychological Science, 28(11), 1547–1562. https://doi.org/10.1177/0956797617723724
Avesani, P., McPherson, B., Hayashi, S., Caiafa, C. F., Henschel, R., Garyfallidis, E., Kitchell, L., Bullock, D., Patterson, A., Olivetti, E., Sporns, O., Saykin, A. J., Wang, L., Dinov, I., Hancock, D., Caron, B., Qian, Y., & Pestilli, F. (2019). The open diffusion data derivatives, brain data upcycling via integrated publishing of derivatives and reproducible open cloud services. Scientific Data, 6(1), 69. https://doi.org/10.1038/s41597-019-0073-y
Blomkvist, A. (2023). Aphantasia: In search of a theory. Mind & Language, 38(3), 866–888. https://doi.org/10.1111/mila.12432
Cohen, R. L. (1984). Individual differences in event memory: A case for nonstrategic factors. Memory & Cognition, 12(6), 633–641. https://doi.org/10.3758/BF03213352
Coltheart, M. (1981). The MRC psycholinguistic database. The Quarterly Journal of Experimental Psychology A: Human Experimental Psychology, 33A, 497–505. https://doi.org/10.1080/14640748108400805
Cox, R. W., Chen, G., Glen, D. R., Reynolds, R. C., & Taylor, P. A. (2017). FMRI Clustering in AFNI: False-positive Rates Redux. Brain Connectivity, 7(3), 152–171. https://doi.org/10.1089/brain.2016.0475
Cui, X., Jeter, C. B., Yang, D., Montague, P. R., & Eagleman, D. M. (2007). Vividness of mental imagery: Individual variability can be measured objectively. Vision Research, 47(4), 474–478. https://doi.org/10.1016/j.visres.2006.11.013
Dijkstra, N., Bosch, S. E., & van Gerven, M. A. J. (2017). Vividness of visual imagery depends on the neural overlap with perception in visual areas. The Journal of Neuroscience, 37(5), 1367–1373. https://doi.org/10.1523/JNEUROSCI.3022-16.2016
Giannou, K., Taylor, J. R., & Lander, K. (2020). Exploring the relationship between mindfulness, compassion and unfamiliar face identification. Journal of Cognitive Psychology, 32(3), 298–322. https://doi.org/10.1080/20445911.2020.1739693
Guerin, S. A., & Miller, M. B. (2009). Lateralization of the parietal old/new effect: An event-related fMRI study comparing recognition memory for words and faces. Neuroimage, 44(1), 232–242. https://doi.org/10.1016/j.neuroimage.2008.08.035
Heavey, C. L., & Hurlburt, R. T. (2008). The phenomena of inner experience. Consciousness and Cognition, 17(3), 798–810. https://doi.org/10.1016/j.concog.2007.12.006
Ishai, A. (2002). Visual imagery of Famous faces: Effects of memory and attention revealed by fMRI. Neuroimage, 17(4), 1729–1741. https://doi.org/10.1006/nimg.2002.1330
Karis, D., Fabiani, M., & Donchin, E. (1984). P300 and memory: Individual differences in the Von Restorff effect. Cognitive Psychology, 16(2), 177–216. https://doi.org/10.1016/0010-0285(84)90007-0
Keogh, R., & Pearson, J. (2018). The blind mind: No sensory visual imagery in aphantasia. Cortex; a Journal Devoted to the Study of the Nervous System and Behavior, 105, 53–60. https://doi.org/10.1016/j.cortex.2017.10.012
Kim, H. (2020). An integrative model of network activity during episodic memory retrieval and a meta-analysis of fMRI studies on source memory retrieval. Brain Research, 1747, 147049. https://doi.org/10.1016/j.brainres.2020.147049
Klimovich-Gray, A., Bozic, M., & Marslen-Wilson, W. D. (2017). Domain-specific and domain-general Processing in Left Perisylvian cortex: Evidence from Russian. Journal of Cognitive Neuroscience, 29(2), 382–397. https://doi.org/10.1162/jocn_a_01047
Li, X., Morgan, P. S., Ashburner, J., Smith, J., & Rorden, C. (2016). The first step for neuroimaging data analysis: DICOM to NIfTI conversion. Journal of Neuroscience Methods, 264, 47–56. https://doi.org/10.1016/j.jneumeth.2016.03.001
Marks, D. F. (1973). Visual imagery differences in the recall of pictures. British Journal of Psychology, 64(1), 17–24.
Megreya, A. M., & Bindemann, M. (2013). Individual differences in personality and face identification. Journal of Cognitive Psychology, 25(1), 30–37. https://doi.org/10.1080/20445911.2012.739153
Milton, F., Fulford, J., Dance, C., Gaddum, J., Heuerman-Williamson, B., Jones, K., Knight, K. F., MacKisack, M., Winlove, C., & Zeman, A. (2021). Behavioral and neural signatures of visual imagery vividness extremes: Aphantasia versus Hyperphantasia. Cerebral Cortex Communications, 2(2), tgab035. https://doi.org/10.1093/texcom/tgab035
Minear, M., & Park, D. C. (2004). A lifespan database of adult facial stimuli. Behavior Research Methods Instruments & Computers, 36(4), 630–633. https://doi.org/10.3758/BF03206543
Mongelli, V., Dehaene, S., Vinckier, F., Peretz, I., Bartolomeo, P., & Cohen, L. (2017). Music and words in the visual cortex: The impact of musical expertise. Cortex; a Journal Devoted to the Study of the Nervous System and Behavior, 86, 260–274. https://doi.org/10.1016/j.cortex.2016.05.016
Monteleone, G. T., Phan, K. L., Nusbaum, H. C., Fitzgerald, D., Irick, J. S., Fienberg, S. E., & Cacioppo, J. T. (2009). Detection of deception using fMRI: Better than chance, but well below perfection. Social Neuroscience, 4(6), 528–538. https://doi.org/10.1080/17470910801903530
Muhmenthaler, M. C., & Meier, B. (2019). Task switching hurts memory encoding. Experimental Psychology, 66(1), 58–67. https://doi.org/10.1027/1618-3169/a000431
Nedergaard, J., & Lupyan, G. (2023). Not Everyone Has an Inner Voice: Behavioral Consequences of Anendophasia. Proceedings of the Annual Meeting of the Cognitive Science Society. https://escholarship.org/uc/item/93p4r8td
Pearson, J. (2019). The human imagination: The cognitive neuroscience of visual mental imagery. Nature Reviews Neuroscience, 20(10). https://doi.org/10.1038/s41583-019-0202-9
Roebuck, H., & Lupyan, G. (2020). The Internal representations Questionnaire: Measuring modes of thinking. Behavior Research Methods, 52(5), 2053–2070. https://doi.org/10.3758/s13428-020-01354-y
Rosenstreich, E., & Ruderman, L. (2016). Not sensitive, yet less biased: A signal detection theory perspective on mindfulness, attention, and recognition memory. Consciousness and Cognition, 43, 48–56. https://doi.org/10.1016/j.concog.2016.05.007
Slotnick, S. D., Thompson, W. L., & Kosslyn, S. M. (2012). Visual memory and visual mental imagery recruit common control and sensory regions of the brain. Cognitive Neuroscience, 3(1), 14–20. https://doi.org/10.1080/17588928.2011.578210
Taylor, R. S., Francis, W. S., Borunda-Vazquez, L., & Carbajal, J. (2019). Mechanisms of word concreteness effects in explicit memory: Does context availability play a role? Memory & Cognition, 47(1), 169–181. https://doi.org/10.3758/s13421-018-0857-x
Tulving, E. (2002). Episodic memory: From mind to Brain. Annu Rev Psychol, 53(1), Article1.
Woodhead, M. M., & Baddeley, A. D. (1981). Individual differences and memory for faces, pictures, and words. Memory & Cognition, 9(4), 368–370. https://doi.org/10.3758/BF03197561
Zerr, C. L., Berg, J. J., Nelson, S. M., Fishell, A. K., Savalia, N. K., & McDermott, K. B. (2018). Learning efficiency: Identifying individual differences in Learning Rate and Retention in healthy adults. Psychological Science, 29(9), 1436–1450. https://doi.org/10.1177/0956797618772540
Funding
This study was supported through Brigham Young University’s “CURA” College Undergraduate Research Award.
Author information
Authors and Affiliations
Contributions
AHS and CBK conceived the research question, conducted analyses, and wrote the manuscript. AHS created the figures. All authors have reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The Institutional Review Board of Brigham Young University approved this study (RB number: X202-067). All patients provided written consent for data collection and publishing, and were screened for MRI safety at Brigham Young University’s MRI facility.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Schmidt, A.H., Kirwan, C.B. Memory retrieval effects as a function of differences in phenomenal experience. Brain Imaging and Behavior (2024). https://doi.org/10.1007/s11682-024-00892-9
Accepted:
Published:
DOI: https://doi.org/10.1007/s11682-024-00892-9