Abstract
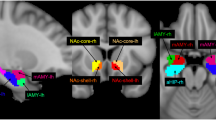
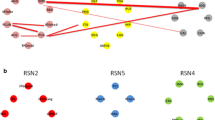
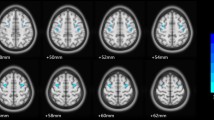
The pathophysiological mechanisms of bipolar disorder (BD) are not completely known, and systemic inflammation and immune dysregulation are considered as risk factors. Previous neuroimaging studies have proved metabolic, structural and functional abnormalities of the amygdala in BD, suggesting the vital role of amygdala in BD patients. This study aimed to test the underlying neural mechanism of inflammation-induced functional connectivity (FC) in the amygdala subregions of BD patients. Resting-state functional MRI (rs-fMRI) was used to delineate the amygdala FC from two pairs of amygdala seed regions (the bilateral lateral and medial amygdala) in 51 unmedicated BD patients and 69 healthy controls (HCs). The levels of pro-inflammatory cytokines including interleukin (IL)-1β, IL-6 and tumor necrosis factor (TNF)-α were measured in the serum. The correlation between abnormal levels of pro-inflammatory cytokines and FC values were calculated in BD patients. The BD group exhibited decreased FC between the right medial amygdala and bilateral medial frontal cortex (MFC), and decreased FC between the left medial amygdala and the left temporal pole (TP), right orbital inferior frontal gyrus compared with HCs. The BD patients had higher levels of TNF-α than HCs. Correlation analysis showed negative correlation between the TNF-α level and abnormal FC of the right medial amygdala-bilateral MFC; and negative correlation between TNF-α levels and abnormal FC of the left medial amygdala-left TP in BD group. These findings suggest that dysfunctional and immune dysregulation between the amygdala and the frontotemporal circuitry might play a critical role in the pathogenesis of BD.



Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Ambrosi, E., Arciniegas, D. B., Madan, A., Curtis, K. N., Patriquin, M. A., Jorge, R. E. … Salas, R. (2017). Insula and amygdala resting-state functional connectivity differentiate bipolar from unipolar depression. Acta Psychiatrica Scandinavica, 136(1), 129–139. https://doi.org/10.1111/acps.12724
Borras-Ferris, L., Perez-Ramirez, U., & Moratal, D. (2019). link-level functional connectivity neuroalterations in autism spectrum disorder: a developmental resting-state fMRI study. Diagnostics (Basel), 9(1). https://doi.org/10.3390/diagnostics9010032
Brietzke, E., Stertz, L., Fernandes, B. S., Kauer-Sant’Anna, M., Mascarenhas, M., Escosteguy, V. A. … Kapczinski, F. (2009). Comparison of cytokine levels in depressed, manic and euthymic patients with bipolar disorder. Journal of Affective Disorders, 116(3), 214–217. https://doi.org/10.1016/j.jad.2008.12.001
Brunoni, A. R., Supasitthumrong, T., Teixeira, A. L., Vieira, E. L., Gattaz, W. F., Bensenor, I. M. … Maes, M. (2020). Differences in the immune-inflammatory profiles of unipolar and bipolar depression. Journal of Affective Disorders, 2628–2615. https://doi.org/10.1016/j.jad.2019.10.037
Bzdok, D., Laird, A. R., Zilles, K., Fox, P. T., & Eickhoff, S. B. (2013). An investigation of the structural, connectional, and functional subspecialization in the human amygdala. Human Brain Mapping, 34(12), 3247–3266. https://doi.org/10.1002/hbm.22138
Capuron, L., & Miller, A. H. (2011). Immune system to brain signaling: neuropsychopharmacological implications. Pharmacology & Therapeutics, 130(2), 226–238. https://doi.org/10.1016/j.pharmthera.2011.01.014
Chen, G., Zhao, L., Jia, Y., Zhong, S., Chen, F., Luo, X. … Wang, Y. (2019a). Abnormal cerebellum-DMN regions connectivity in unmedicated bipolar II disorder. Journal of Affective Disorders, 243441–243447. https://doi.org/10.1016/j.jad.2018.09.076
Chen, M. H., Chang, W. C., Hsu, J. W., Huang, K. L., Tu, P. C., Su, T. P. … Bai, Y. M. (2019b). Correlation of proinflammatory cytokines levels and reduced gray matter volumes between patients with bipolar disorder and unipolar depression. Journal of Affective Disorders, 2458–2415. https://doi.org/10.1016/j.jad.2018.10.106
Davies, K. A., Cooper, E., Voon, V., Tibble, J., Cercignani, M., & Harrison, N. A. (2020). Interferon and anti-TNF therapies differentially modulate amygdala reactivity which predicts associated bidirectional changes in depressive symptoms. Molecular Psychiatry. https://doi.org/10.1038/s41380-020-0790-9
Deco, G., & Kringelbach, M. L. (2014). Great expectations: using whole-brain computational connectomics for understanding neuropsychiatric disorders. Neuron, 84(5), 892–905. https://doi.org/10.1016/j.neuron.2014.08.034
Dickerson, F., Severance, E., & Yolken, R. (2017). The microbiome, immunity, and schizophrenia and bipolar disorder. Brain, Behavior, and Immunity, 6246–6252. https://doi.org/10.1016/j.bbi.2016.12.010
Dinarello, C. A. (2000). Proinflammatory cytokines. Chest, 118(2), 503–508. https://doi.org/10.1378/chest.118.2.503
Fan, C., Song, Q., Wang, P., Li, Y., Yang, M., Liu, B., & Yu, S. Y. (2018). Curcumin protects against chronic stress-induced dysregulation of neuroplasticity and depression-like behaviors via suppressing IL-1beta pathway in rats. Neuroscience, 39292–39106. https://doi.org/10.1016/j.neuroscience.2018.09.028
Fan, L., Li, H., Zhuo, J., Zhang, Y., Wang, J., Chen, L. … Jiang, T. (2016). The Human Brainnetome Atlas: a new brain atlas based on connectional architecture. Cerebal Cortex, 26(8), 3508–3526. https://doi.org/10.1093/cercor/bhw157
Felger, J. C. (2018). Imaging the role of inflammation in mood and anxiety-related disorders. Curr Neuropharmacol, 16(5), 533–558. https://doi.org/10.2174/1570159X15666171123201142
Fiedorowicz, J. G., Prossin, A. R., Johnson, C. P., Christensen, G. E., Magnotta, V. A., & Wemmie, J. A. (2015). Peripheral inflammation during abnormal mood states in bipolar I disorder. Journal of Affective Disorders, 187172–187178. https://doi.org/10.1016/j.jad.2015.08.036
Fox, M. D., Zhang, D., Snyder, A. Z., & Raichle, M. E. (2009). The global signal and observed anticorrelated resting state brain networks. Journal of Neurophysiology, 101(6), 3270–3283. https://doi.org/10.1152/jn.90777.2008
Friston, K. J., Williams, S., Howard, R., Frackowiak, R. S., & Turner, R. (1996). Movement-related effects in fMRI time-series. Magnetic Resonance in Medicine, 35(3), 346–355. https://doi.org/10.1002/mrm.1910350312
Goldman, D. A., Sankar, A., Colic, L., Villa, L., Kim, J. A., Pittman, B. … Blumberg, H. P. (2021). A graph theory-based whole brain approach to assess mood state differences in adolescents and young adults with bipolar disorder. Bipolar Disorders. https://doi.org/10.1111/bdi.13144
Goldsmith, D. R., Rapaport, M. H., & Miller, B. J. (2016). A meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. Molecular Psychiatry, 21(12), 1696–1709. https://doi.org/10.1038/mp.2016.3
Haapakoski, R., Mathieu, J., Ebmeier, K. P., Alenius, H., & Kivimaki, M. (2015). Cumulative meta-analysis of interleukins 6 and 1beta, tumour necrosis factor alpha and C-reactive protein in patients with major depressive disorder. Brain, Behavior, and Immunity, 49206–49215. https://doi.org/10.1016/j.bbi.2015.06.001
Ho, N. F., Li, H. C. P., Lee, D. R., Chew, Q. H., Chen, G., & Sim, K. (2019). The amygdala in schizophrenia and bipolar disorder: a synthesis of structural MRI, diffusion tensor imaging, and resting-state functional connectivity findings. Harvard Review of Psychiatry, 27(3), 150–164. https://doi.org/10.1097/HRP.0000000000000207
Isgren, A., Sellgren, C., Ekman, C. J., Holmen-Larsson, J., Blennow, K., Zetterberg, H. … Landen, M. (2017). Markers of neuroinflammation and neuronal injury in bipolar disorder: Relation to prospective clinical outcomes. Brain, Behavior, and Immunity, 65195–65201. https://doi.org/10.1016/j.bbi.2017.05.002
Jalbrzikowski, M., Larsen, B., Hallquist, M. N., Foran, W., Calabro, F., & Luna, B. (2017). Development of white matter microstructure and intrinsic functional connectivity between the amygdala and ventromedial prefrontal cortex: associations with anxiety and depression. Biological Psychiatry, 82(7), 511–521. https://doi.org/10.1016/j.biopsych.2017.01.008
Janak, P. H., & Tye, K. M. (2015). From circuits to behaviour in the amygdala. Nature, 517(7534), 284–292. https://doi.org/10.1038/nature14188
Jenkinson, M., Bannister, P., Brady, M., & Smith, S. (2002). Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage, 17(2), 825–841. https://doi.org/10.1016/s1053-8119(02)91132-8
Jesudas, B. R., Nandeesha, H., Menon, V., & Allimuthu, P. (2019). Relationship of elevated neural cell adhesion molecule 1 with interleukin-10 and disease severity in bipolar disorder. Asian Journal of Psychiatry, 47101849. https://doi.org/10.1016/j.ajp.2019.101849
Johnston, J., Wang, F., Liu, J., Blond, B. N., Wallace, A., Liu, J. … Blumberg, H. P. (2017). Multimodal neuroimaging of frontolimbic structure and function associated with suicide attempts in adolescents and young adults with bipolar disorder. The American Journal of Psychiatry, 174(7), 667–675. https://doi.org/10.1176/appi.ajp.2016.15050652
Karabulut, S., Tasdemir, I., Akcan, U., Kucukali, C. I., Tuzun, E., & Cakir, S. (2019). Inflammation and neurodegeneration in patients with early-Stageand chronic bipolar disorder. Türk Psikiyatri Dergisi, 30(2), 75–81
Kasai, K., Shenton, M. E., Salisbury, D. F., Onitsuka, T., Toner, S. K., Yurgelun-Todd, D. … McCarley, R. W. (2003). Differences and similarities in insular and temporal pole MRI gray matter volume abnormalities in first-episode schizophrenia and affective psychosis. Archives Of General Psychiatry, 60(11), 1069–1077. https://doi.org/10.1001/archpsyc.60.11.1069
Kupfer, D. J. (2005). The increasing medical burden in bipolar disorder. JAMA, 293(20), 2528–2530. https://doi.org/10.1001/jama.293.20.2528
Li, G., Liu, P., Andari, E., Zhang, A., & Zhang, K. (2018). The role of amygdala in patients with euthymic bipolar disorder during resting state. Frontiers in Psychiatry, 9445, https://doi.org/10.3389/fpsyt.2018.00445
Lin, K., Shao, R., Geng, X., Chen, K., Lu, R., Gao, Y. … So, K. F. (2018). Illness, at-risk and resilience neural markers of early-stage bipolar disorder. Journal of Affective Disorders, 23816–23823. https://doi.org/10.1016/j.jad.2018.05.017
Lin, K., Shao, R., Wang, R., Lu, W., Zou, W., Chen, K. … So, K. F. (2020). Inflammation, brain structure and cognition interrelations among individuals with differential risks for bipolar disorder. Brain, Behavior, and Immunity, 83192–83199. https://doi.org/10.1016/j.bbi.2019.10.010
Liu, K., Zhao, X., Lu, X., Zhu, X., Chen, H., Wang, M., ... Lv, Z. (2019). Effect of selective serotonin reuptake inhibitor on prefrontal-striatal connectivity is dependent on the level of TNF-alpha in patients with major depressive disorder. Psychological Medicine, 49(15), 2608–2616. https://doi.org/10.1017/S0033291718003616
Liu, T. Y., Chen, Y. S., Su, T. P., Hsieh, J. C., & Chen, L. F. (2014). Abnormal early gamma responses to emotional faces differentiate unipolar from bipolar disorder patients. BioMed Research International, 2014906104, https://doi.org/10.1155/2014/906104
McIntosh, R. C., Paul, R., Ndhlovu, L. C., Hidalgo, M., Lobo, J. D., Walker, M. … Kallianpur, K. J. (2018). Resting-state connectivity and spontaneous activity of ventromedial prefrontal cortex predict depressive symptomology and peripheral inflammation in HIV. The Journal of NeuroVirology, 24(5), 616–628. https://doi.org/10.1007/s13365-018-0658-9
Mehta, N. D., Haroon, E., Xu, X., Woolwine, B. J., Li, Z., & Felger, J. C. (2018). Inflammation negatively correlates with amygdala-ventromedial prefrontal functional connectivity in association with anxiety in patients with depression: Preliminary results. Brain, Behavior, and Immunity, 73725–73730. https://doi.org/10.1016/j.bbi.2018.07.026
Modabbernia, A., Taslimi, S., Brietzke, E., & Ashrafi, M. (2013). Cytokine alterations in bipolar disorder: a meta-analysis of 30 studies. Biological Psychiatry, 74(1), 15–25. https://doi.org/10.1016/j.biopsych.2013.01.007
Mosher, C. P., Zimmerman, P. E., & Gothard, K. M. (2010). Response characteristics of basolateral and centromedial neurons in the primate amygdala. The Journal of Neuroscience, 30(48), 16197–16207. https://doi.org/10.1523/JNEUROSCI.3225-10.2010
Mukherjee, P., Sabharwal, A., Kotov, R., Szekely, A., Parsey, R., Barch, D. M., & Mohanty, A. (2016). Disconnection between amygdala and medial prefrontal cortex in psychotic disorders. Schizophrenia Bulletin, 42(4), 1056–1067. https://doi.org/10.1093/schbul/sbw012
Mulders, P. C., van Eijndhoven, P. F., Schene, A. H., Beckmann, C. F., & Tendolkar, I. (2015). Resting-state functional connectivity in major depressive disorder: A review. Neuroscience & Biobehavioral Reviews, 56330–56344. https://doi.org/10.1016/j.neubiorev.2015.07.014
Muscatell, K. A., Dedovic, K., Slavich, G. M., Jarcho, M. R., Breen, E. C., Bower, J. E. … Eisenberger, N. I. (2015). Greater amygdala activity and dorsomedial prefrontal-amygdala coupling are associated with enhanced inflammatory responses to stress. Brain, Behavior, and Immunity, 4346–4353. https://doi.org/10.1016/j.bbi.2014.06.201
Najjar, S., Pearlman, D. M., Alper, K., Najjar, A., & Devinsky, O. (2013). Neuroinflammation and psychiatric illness. Journal of Neuroinflammation, 1043, https://doi.org/10.1186/1742-2094-10-43
Neves, M. C., Albuquerque, M. R., Malloy-Diniz, L., Nicolato, R., Silva, N. F., de Souza-Duran, F. L. … Correa, H. (2015). A voxel-based morphometry study of gray matter correlates of facial emotion recognition in bipolar disorder. Psychiatry Research, 233(2), 158–164. https://doi.org/10.1016/j.pscychresns.2015.05.009
Niu, Z., Yang, L., Wu, X., Zhu, Y., Chen, J., & Fang, Y. (2019). The relationship between neuroimmunity and bipolar disorder: mechanism and translational application. Neuroscience Bulletin, 35(4), 595–607. https://doi.org/10.1007/s12264-019-00403-7
Ochsner, K. N., Ray, R. R., Hughes, B., McRae, K., Cooper, J. C., Weber, J. … Gross, J. J. (2009). Bottom-up and top-down processes in emotion generation: common and distinct neural mechanisms. Psychological Science, 20(11), 1322–1331. https://doi.org/10.1111/j.1467-9280.2009.02459.x
Olson, I. R., Plotzker, A., & Ezzyat, Y. (2007). The Enigmatic temporal pole: a review of findings on social and emotional processing. Brain, 130(Pt 7), 1718–1731. https://doi.org/10.1093/brain/awm052
Pan, A. Y., Ryu, E., Geske, J. R., Zhou, X. Y., McElroy, S. L., Cicek, M. S. … Andreazza, A. C. (2020). The impact of sample processing on inflammatory markers in serum: Lessons learned. World Journal of Biological Psychiatry, 21(3), 230–237. https://doi.org/10.1080/15622975.2019.1696474
Pantovic-Stefanovic, M., Petronijevic, N., Dunjic-Kostic, B., Velimirovic, M., Nikolic, T., Jurisic, V. … Ivkovic, M. (2018). sVCAM-1, sICAM-1, TNF-alpha and IL-6 levels in bipolar disorder type I: Acute, longitudinal and therapeutic implications. World Journal of Biological Psychiatry, 19(sup2):S41-S51. https://doi.org/10.1080/15622975.2016.1259498
Pape, H. C., & Pare, D. (2010). Plastic synaptic networks of the amygdala for the acquisition, expression, and extinction of conditioned fear. Physiological Reviews, 90(2), 419–463. https://doi.org/10.1152/physrev.00037.2009
Rajkowska, G., Halaris, A., & Selemon, L. D. (2001). Reductions in neuronal and glial density characterize the dorsolateral prefrontal cortex in bipolar disorder. Biological Psychiatry, 49(9), 741–752. https://doi.org/10.1016/s0006-3223(01)01080-0
Rosenblat, J. D., & McIntyre, R. S. (2016). Bipolar Disorder and Inflammation. Psychiatric Clinics of North America, 39(1), 125–137. https://doi.org/10.1016/j.psc.2015.09.006
Rowland, T., Perry, B. I., Upthegrove, R., Barnes, N., Chatterjee, J., Gallacher, D., & Marwaha, S. (2018). Neurotrophins, cytokines, oxidative stress mediators and mood state in bipolar disorder: systematic review and meta-analyses. British Journal of Psychiatry, 213(3), 514–525. https://doi.org/10.1192/bjp.2018.144
Roy, A. K., Shehzad, Z., Margulies, D. S., Kelly, A. M., Uddin, L. Q., Gotimer, K. … Milham, M. P. (2009). Functional connectivity of the human amygdala using resting state fMRI. Neuroimage, 45(2), 614–626. https://doi.org/10.1016/j.neuroimage.2008.11.030
Savitz, J. B., Price, J. L., & Drevets, W. C. (2014). Neuropathological and neuromorphometric abnormalities in bipolar disorder: view from the medial prefrontal cortical network. Neuroscience and Biobehavioral Reviews, 42132–42147. https://doi.org/10.1016/j.neubiorev.2014.02.008
Sayana, P., Colpo, G. D., Simoes, L. R., Giridharan, V. V., Teixeira, A. L., Quevedo, J., & Barichello, T. (2017). A systematic review of evidence for the role of inflammatory biomarkers in bipolar patients. Journal of Psychiatric Research, 92160–92182. https://doi.org/10.1016/j.jpsychires.2017.03.018
Spuhler, K., Bartlett, E., Ding, J., DeLorenzo, C., Parsey, R., & Huang, C. (2018). Diffusion entropy: a potential neuroimaging biomarker of bipolar disorder in the temporal pole. Synapse, 72(2), https://doi.org/10.1002/syn.22015
Tang, Y., Ma, Y., Chen, X., Fan, X., Jiang, X., Zhou, Y. … Wei, S. (2018). Age-specific effects of structural and functional connectivity in prefrontal-amygdala circuitry in women with bipolar disorder. BMC Psychiatry, 18(1), 177. https://doi.org/10.1186/s12888-018-1732-9
Tseng, W. L., Thomas, L. A., Harkins, E., Stoddard, J., Zarate, C. J., Pine, D. S. … Brotman, M. A. (2016). Functional connectivity during masked and unmasked face emotion processing in bipolar disorder. Psychiatry Research: Neuroimaging, 2581–2589. https://doi.org/10.1016/j.pscychresns.2016.10.006
Tsujii, N., Mikawa, W., Adachi, T., Hirose, T., & Shirakawa, O. (2018). Shared and differential cortical functional abnormalities associated with inhibitory control in patients with schizophrenia and bipolar disorder. Scientific Reports, 8(1), 4686. https://doi.org/10.1038/s41598-018-22929-y
Tu, P. C., Li, C. T., Lin, W. C., Chen, M. H., Su, T. P., & Bai, Y. M. (2017). Structural and functional correlates of serum soluble IL-6 receptor level in patients with bipolar disorder. Journal of Affective Disorders, 219172–219177. https://doi.org/10.1016/j.jad.2017.04.036
Vai, B., Poletti, S., Radaelli, D., Dallaspezia, S., Bulgarelli, C., Locatelli, C. … Benedetti, F. (2015). Successful antidepressant chronotherapeutics enhance fronto-limbic neural responses and connectivity in bipolar depression. Psychiatry Research, 233(2), 243–253. https://doi.org/10.1016/j.pscychresns.2015.07.015
Vai, B., Serretti, A., Poletti, S., Mascia, M., Lorenzi, C., Colombo, C., & Benedetti, F. (2020). Cortico-limbic functional connectivity mediates the effect of early life stress on suicidality in bipolar depressed 5-HTTLPR*s carriers. Journal of Affective Disorders, 263420–263427. https://doi.org/10.1016/j.jad.2019.11.142
Van der Schot, A., Kahn, R., Ramsey, N., Nolen, W., & Vink, M. (2010). Trait and state dependent functional impairments in bipolar disorder. Psychiatry Research, 184(3), 135–142. https://doi.org/10.1016/j.pscychresns.2010.07.009
Van Dijk, K. R., Sabuncu, M. R., & Buckner, R. L. (2012). The influence of head motion on intrinsic functional connectivity MRI. Neuroimage, 59(1), 431–438. https://doi.org/10.1016/j.neuroimage.2011.07.044
Vieta, E., Berk, M., Schulze, T. G., Carvalho, A. F., Suppes, T., Calabrese, J. R. … Grande, I. (2018). Bipolar disorders. Nature Reviews Disease Primers, 418008. https://doi.org/10.1038/nrdp.2018.8
Wang, Y., Zhong, S., Jia, Y., Sun, Y., Wang, B., Liu, T. … Huang, L. (2016). Disrupted resting-state functional connectivity in nonmedicated bipolar disorder. Radiology, 280(2), 529–536. https://doi.org/10.1148/radiol.2016151641
Wang, Y., Zhong, S., Jia, Y., Zhou, Z., Zhou, Q., & Huang, L. (2015). Reduced interhemispheric resting-state functional connectivity in unmedicated bipolar II disorder. Acta Psychiatrica Scandinavica, 132(5), 400–407. https://doi.org/10.1111/acps.12429
Wilczynska, K., Simonienko, K., Konarzewska, B., Szajda, S. D., & Waszkiewicz, N. (2018). Morphological changes of the brain in mood disorders. Psychiatria Polska, 52(5), 797–805. https://doi.org/10.12740/PP/89553
Wu, Y., Li, H., Zhou, Y., Yu, J., Zhang, Y., Song, M. … Jiang, T. (2016). Sex-specific neural circuits of emotion regulation in the centromedial amygdala. Scientific Reports, 623112, https://doi.org/10.1038/srep23112
Yan, C. G., Cheung, B., Kelly, C., Colcombe, S., Craddock, R. C., Di Martino, A. … Milham, M. P. (2013a). A comprehensive assessment of regional variation in the impact of head micromovements on functional connectomics. Neuroimage, 76183–76201. https://doi.org/10.1016/j.neuroimage.2013.03.004
Yan, C. G., Craddock, R. C., Zuo, X. N., Zang, Y. F., & Milham, M. P. (2013b). Standardizing the intrinsic brain: towards robust measurement of inter-individual variation in 1000 functional connectomes. Neuroimage, 80246–80262. https://doi.org/10.1016/j.neuroimage.2013.04.081
Yan, C. G., Wang, X. D., Zuo, X. N., & Zang, Y. F. (2016). DPABI: Data Processing& Analysis for (Resting-State) Brain Imaging. Neuroinformatics, 14(3), 339-351. https://doi.org/10.1007/s12021-016-9299-4
Yin, Z., Chang, M., Wei, S., Jiang, X., Zhou, Y., Cui, L. … Tang, Y. (2018). Decreased functional connectivity in insular subregions in depressive episodes of bipolar disorder and major depressive disorder. Frontiers in Neuroscience, 12842, https://doi.org/10.3389/fnins.2018.00842
Yu, H. L., Liu, W. B., Wang, T., Huang, P. Y., Jie, L. Y., Sun, J. Z. … Zhang, M. M. (2017). Difference in resting-state fractional amplitude of low-frequency fluctuation between bipolar depression and unipolar depression patients. European Review for Medical and Pharmacological Sciences, 21(7), 1541–1550
Yu, H., Meng, Y. J., Li, X. J., Zhang, C., Liang, S., Li, M. L. … Li, T. (2019). Common and distinct patterns of grey matter alterations in borderline personality disorder and bipolar disorder: voxel-based meta-analysis. British Journal of Psychiatry, 215(1), 395–403. https://doi.org/10.1192/bjp.2019.44
Zhang, L., Opmeer, E. M., van der Meer, L., Aleman, A., Curcic-Blake, B., & Ruhe, H. G. (2018a). Altered frontal-amygdala effective connectivity during effortful emotion regulation in bipolar disorder. Bipolar Disorder, 20(4), 349–358. https://doi.org/10.1111/bdi.12611
Zhang, L., Wu, H., Xu, J., & Shang, J. (2018b). Abnormal global functional connectivity patterns in medication-free major depressive disorder. Frontiers in Neuroscience, 12692, https://doi.org/10.3389/fnins.2018.00692
Zhong, Y., Wang, C., Gao, W., Xiao, Q., Lu, D., Jiao, Q. … Lu, G. (2019). Aberrant Resting-State Functional Connectivity in the Default Mode Network in Pediatric Bipolar Disorder Patients with and without Psychotic Symptoms. Neurosci Bull, 35(4), 581–590. https://doi.org/10.1007/s12264-018-0315-6
Zhou, Q., Womer, F. Y., Kong, L., Wu, F., Jiang, X., Zhou, Y. … Wang, F. (2017). Trait-related cortical-subcortical dissociation in bipolar disorder: analysis of network degree centrality. The Journal of Clinical Psychiatry, 78(5), 584–591. https://doi.org/10.4088/JCP.15m10091
Zuliani, R., Moorhead, T. W., Job, D., McKirdy, J., Sussmann, J. E., Johnstone, E. C. … McIntosh, A. M. (2009). Genetic variation in the G72 (DAOA) gene affects temporal lobe and amygdala structure in subjects affected by bipolar disorder. Bipolar Disorder, 11(6), 621–627. https://doi.org/10.1111/j.1399-5618.2009.00731.x
Funding
The study was supported by grants from the National Natural Science Foundation of China (81671670, 81971597 and 82102003); Project in Basic Research and Applied Basic Research in General Colleges and Universities of Guangdong, China (2018KZDXM009); National Key Research and Development Project (2020YFC2005700). The funding organizations play no further role in study design, data collection, analysis and interpretation and paper writing.
Author information
Authors and Affiliations
Contributions
Ying Wang designed the study; Jiaying Gong, Ying Wang contributed to data sources and study selection; Jiaying Gong, Guanmao Chen, Feng Chen, Shuming Zhong, Pan Chen, Hui Zhong, Shunkai Lai, Guixian Tang, Jurong Wang, Zhenye Luo, Zhangzhang Qi, Yanbin Jia contributed to data acquisition; Guanmao Chen, Feng Chen contributed to data analysis; Jiaying Gong wrote the manuscript; Pan Chen, Feng Chen, Jiaying Gong, Guanmao Chen, Feng Chen, Hui Zhong, Li Huang, Ying Wang revised the manuscript. All authors contributed to and have approved the final manuscript. We thank all the authors of the included studies who responded to our requests for further information.
Corresponding author
Ethics declarations
Ethical approval
This study was approved by the Ethics Committee of First Affiliated Hospital of Jinan University, Guangzhou, China.
Consent to participate
All subjects signed a written informed consent form after full writing and verbal explanation of the study.
Consent to publish
All authors contributed to and have approved the submission and publishment of final manuscript.
Conflict of interest
The authors have declared that no competing interest exists.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 5.65 MB)
Rights and permissions
About this article
Cite this article
Gong, J., Chen, G., Chen, F. et al. Association between resting-state functional connectivity of amygdala subregions and peripheral pro-inflammation cytokines levels in bipolar disorder. Brain Imaging and Behavior 16, 1614–1626 (2022). https://doi.org/10.1007/s11682-022-00636-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11682-022-00636-7