Abstract
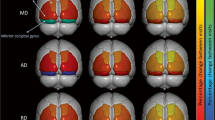
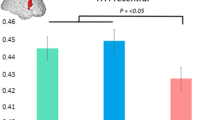
This paper investigated cortical folding in Huntington’s disease to understand how disease progression impacts the surface of the cortex. Cortical morphometry changes in eight gyral based regions of interest (i.e. the left and right hemispheres of the lateral occipital, precentral, superior frontal and rostral middle gyri) were examined. We used existing neuroimaging data from IMAGE-HD, comprising 26 pre-symptomatic, 26 symptomatic and 24 healthy control individuals at three separate time points (baseline, 18-month, 30-month). Local gyrification index and cortical thickness were derived as the measures of cortical morphometry using FreeSurfer 6.0’s longitudinal pipeline. The gyral based regions of interest were identified using the Desikan-Killiany Atlas. A Group by Time repeated measures ANCOVA was conducted for each region of interest. We found significantly lower LGI at a group level in the right hemisphere lateral occipital region and both hemispheres of the precentral region; as well as significantly reduced cortical thickness at a group level in both hemispheres of the lateral occipital and precentral regions and the right hemisphere of the superior frontal region. We also found a Group by Time interaction for Local gyrification index in the right hemisphere lateral occipital region. This change was largely driven by a significant decrease in the symptomatic group between baseline and 18-months. Additionally, lower local gyrification index and cortical thickness were associated with higher disease burden score. These findings demonstrate that significant longitudinal decline in right hemisphere local gyrification index is evident during manifest disease in lateral occipital cortex and that these changes are more profound in individuals with greater disease burden score.



Similar content being viewed by others
Data availability
All data and materials were collected as part of the IMAGE-HD study and are available upon request.
References
Babcock, D. T., & Ganetzky, B. (2015). Transcellular spreading of huntingtin aggregates in the Drosophila brain. Proceedings of the National Academy of Sciences, 112(39), E5427–E5433. https://doi.org/10.1073/pnas.1516217112
Blanken, L. M. E., Mous, S. E., Ghassabian, A., Muetzel, R. L., Schoemaker, N. K., Marroun, E. … Verhulst, F. C. (2015). Cortical morphology in 6-to 10-year old children with autistic traits: a population-based neuroimaging study. American Journal of Psychiatry, 172(5), 479–486
Dale, A. M., Fischl, B., & Sereno, M. I. (1999). Cortical surface-based analysis: I. Segmentation and surface reconstruction. Neuroimage, 9(2), 179–194
Desikan, R. S., Ségonne, F., Fischl, B., Quinn, B. T., Dickerson, B. C., Blacker, D. … Hyman, B. T. (2006). An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage, 31(3), 968–980
Domínguez, D., Egan, J. F., Gray, G. F., Poudel, M. A., Churchyard, G. R., Chua, A. … Georgiou-Karistianis, N. (2013). Multi-modal neuroimaging in premanifest and early huntington’s disease: 18 month longitudinal data from the IMAGE-HD Study. PLoS ONE, 8(9), e74131. https://doi.org/10.1371/journal.pone.0074131
Domínguez, J. F., Stout, J. C., Poudel, G., Churchyard, A., Chua, P., Egan, G. F., & Georgiou-Karistianis, N. (2016). Multimodal imaging biomarkers in premanifest and early Huntington’s disease: 30-month IMAGE-HD data. The British Journal of Psychiatry, 208(6), 571–578
Douaud, G., Gaura, V., Ribeiro, M. J., Lethimonnier, F., Maroy, R., Verny, C. … Hantraye, P. (2006). Distribution of grey matter atrophy in Huntington’s disease patients: a combined ROI-based and voxel-based morphometric study. Neuroimage, 32(4), 1562–1575
Fischl, B., & Dale, A. M. (2000). Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proceedings of the National Academy of Sciences, 97(20), 11050–11055. https://doi.org/10.1073/pnas.200033797
Fischl, B., Sereno, M. I., & Dale, A. M. (1999). Cortical surface-based analysis: II: inflation, flattening, and a surface-based coordinate system. Neuroimage, 9(2), 195–207
Georgiou-Karistianis, N., Gray, M. A., Dymowski, A. R., Bohanna, I., Johnston, L. A., Churchyard, A. … Egan, G. F. (2013a). Automated differentiation of pre-diagnosis Huntington’s disease from healthy control individuals based on quadratic discriminant analysis of the basal ganglia: the IMAGE-HD study. Neurobiology of Disease, 51, 82–92
Georgiou-Karistianis, N., Scahill, R., Tabrizi, S. J., Squitieri, F., & Aylward, E. (2013). Structural MRI in Huntington’s disease and recommendations for its potential use in clinical trials. Neuroscience and Biobehavioral Reviews, 37(3), 480–490
Georgiou-Karistianis, N., Sritharan, A., Asadi, H., Johnston, L., Churchyard, A., & Egan, G. (2011). Diffusion tensor imaging in Huntington’s disease reveals distinct patterns of white matter degeneration associated with motor and cognitive deficits. Brain Imaging and Behavior, 5(3), 171–180
Georgiou‐Karistianis, Nellie, et al. (2014). Functional magnetic resonance imaging of working memory in Huntington's disease: cross‐sectional data from the IMAGE‐HD study. Human Brain Mapping 35.5, 1847–1864.
Im, K., Lee, J. M., Seo, S. W., Kim, S. H. I., Kim, S. H. I., & Na, D. L. (2008). Sulcal morphology changes and their relationship with cortical thickness and gyral white matter volume in mild cognitive impairment and Alzheimer’s disease. Neuroimage, 43(1), 103–113
Johnson, E. B., Ziegler, G., Penny, W., Rees, G., Tabrizi, S. J., Scahill, R. I., & Gregory, S. (2019). Dynamics of cortical degeneration over a decade in Huntington’s Disease. BioRxiv, 537977. https://doi.org/10.1101/537977
Jubault, T., Gagnon, J. F., Karama, S., Ptito, A., Lafontaine, A. L., Evans, A. C., & Monchi, O. (2011). Patterns of cortical thickness and surface area in early Parkinson’s disease. Neuroimage, 55(2), 462–467
Kubera, K. M., Schmitgen, M. M., Hirjak, D., Wolf, R. C., & Orth, M. (2019). Cortical neurodevelopment in pre-manifest Huntington’s disease. NeuroImage: Clinical, 101913
Lee, J. K., Mathews, K., Schlaggar, B., Perlmutter, J., Paulsen, J. S., Epping, E. … Nopoulos, P. (2012). Measures of growth in children at risk for Huntington disease. Neurology, 79(7), 668 LP – 674. https://doi.org/10.1212/WNL.0b013e3182648b65
Libero, L. E., Schaer, M., Li, D. D., Amaral, D. G., & Nordahl, C. W. (2019). A longitudinal study of local gyrification index in young boys with autism spectrum disorder. Cerebral Cortex, 29(6), 2575–2587
Mangin, J. F., Rivière, D., Duchesnay, E., Cointepas, Y., Gaura, V., Verny, C. … Hantraye, P. (2020). Neocortical morphometry in Huntington’s disease: Indication of the coexistence of abnormal neurodevelopmental and neurodegenerative processes. NeuroImage: Clinical, 26, 102211
Marder, K., & Mehler, M. F. (2012). Development and neurodegeneration: turning HD pathogenesis on its head. AAN Enterprises
McColgan, P., Seunarine, K. K., Gregory, S., Razi, A., Papoutsi, M., Long, J. D. … Roos, R. A. C. (2017). Topological length of white matter connections predicts their rate of atrophy in premanifest Huntington’s disease. JCI Insight, 2, 8
Mehler, M. F., & Gokhan, S. (2000). Mechanisms underlying neural cell death in neurodegenerative diseases: alterations of a developmentally-mediated cellular rheostat. Trends in Neurosciences, 23(12), 599–605
Molero, A. E., Gokhan, S., Gonzalez, S., Feig, J. L., Alexandre, L. C., & Mehler, M. F. (2009). Impairment of developmental stem cell-mediated striatal neurogenesis and pluripotency genes in a knock-in model of Huntington’s disease. Proceedings of the National Academy of Sciences, 106(51), 21900–21905
Nopoulos, Magnotta, V. A., Mikos, A., Paulson, H., Andreasen, N. C., Paulsen, J. S., Nopoulos, P. C. … Paulsen, J. S. (2007). Morphology of the cerebral cortex in preclinical Huntington’s disease. American Journal of Psychiatry, 164(9), 1428–1434
Nopoulos, P. C. (2016). Huntington disease: a single-gene degenerative disorder of the striatum. Dialogues in Clinical Neuroscience, 18(1), 91
Nordahl, C. W., Dierker, D., Mostafavi, I., Schumann, C. M., Rivera, S. M., Amaral, D. G., & Van Essen, D. C. (2007). Cortical folding abnormalities in autism revealed by surface-based morphometry. Journal of Neuroscience, 27(43), 11725–11735
Paulsen, J. S., Magnotta, V. A., Mikos, A. E., Paulson, H. L., Penziner, E., Andreasen, N. C., & Nopoulos, P. C. (2006). Brain structure in preclinical Huntington’s disease. Biological Psychiatry, 59(1), 57–63
Pereira, J. B., Ibarretxe-Bilbao, N., Marti, M., Compta, Y., Junqué, C., Bargallo, N., & Tolosa, E. (2012). Assessment of cortical degeneration in patients with Parkinson’s disease by voxel‐based morphometry, cortical folding, and cortical thickness. Human Brain Mapping, 33(11), 2521–2534
Plocharski, M., Østergaard, L. R., & Initiative, A. D. N. (2016). Extraction of sulcal medial surface and classification of Alzheimer’s disease using sulcal features. Computer Methods and Programs in Biomedicine, 133, 35–44
Poudel, G., Stout, J. C., Gray, M., Chua, P., Borowsky, B., Egan, G. F., & Georgiou-Karistianis, N. (2017). Longitudinal changes in the fronto-striatal network are associated with executive dysfunction and behavioral dysregulation in Huntington’s disease: 30 months IMAGE-HD data. Cortex, 92, 139–149
Poudel, G. R., Harding, I. H., Egan, G. F., & Georgiou-Karistianis, N. (2019). Network spread determines severity of degeneration and disconnection in Huntington’s disease. Human Brain Mapping. https://doi.org/10.1002/hbm.24695
Poudel, G. R., Stout, J. C., Salmon, L., Churchyard, A., Chua, P., Georgiou-Karistianis, N., & Egan, G. F. (2014). White matter connectivity reflects clinical and cognitive status in Huntington’s disease. Neurobiology of Disease, 65, 180–187
Reuter, M., & Fischl, B. (2011). Avoiding asymmetry-induced bias in longitudinal image processing. Neuroimage, 57(1), 19–21
Reuter, M., Rosas, H. D., & Fischl, B. (2012a). Longitudinal FreeSurfer for reliable imaging biomarkers. NIBAD’12, 275
Reuter, M., Schmansky, N. J., Rosas, H. D., & Fischl, B. (2012). Within-subject template estimation for unbiased longitudinal image analysis. NeuroImage, 61(4), 1402–1418. https://doi.org/10.1016/j.neuroimage.2012.02.084
Rosas, H. D., Hevelone, N. D., Zaleta, A. K., Greve, D. N., Salat, D. H., & Fischl, B. (2005). Regional cortical thinning in preclinical Huntington disease and its relationship to cognition. Neurology, 65(5), 745–747
Rosas, H. D., Liu, A. K., Hersch, S., Glessner, M., Ferrante, R. J., Salat, D. H. … Fischl, B. (2002). Regional and progressive thinning of the cortical ribbon in Huntington’s disease. Neurology, 58(5), 695–701
Ross, C. A., Aylward, E. H., Wild, E. J., Langbehn, D. R., Long, J. D., Warner, J. H. … Paulsen, J. S. (2014). Huntington disease: natural history, biomarkers and prospects for therapeutics. Nature Reviews Neurology, 10(4), 204
Schaer, M., Cuadra, M. B., Schmansky, N., Fischl, B., Thiran, J. P., & Eliez, S. (2012). How to measure cortical folding from MR images: a step-by-step tutorial to compute local gyrification index. JoVE (Journal of Visualized Experiments), 59, e3417
Schaer, M., Cuadra, M. B., Tamarit, L., Lazeyras, F., Eliez, S., & Thiran, J. P. (2008). A surface-based approach to quantify local cortical gyrification. IEEE Transactions on Medical Imaging, 27(2), 161–170
Shishegar, R., Manton, J. H., Walker, D. W., Britto, J. M., & Johnston, L. A. (2015). Quantifying gyrification using Laplace Beltrami eigenfunction level-sets. 2015 IEEE 12th International Symposium on Biomedical Imaging (ISBI), 1272–1275
Shishegar, R., Pizzagalli, F., Georgiou-Karistianis, N., Egan, G. F., Jahanshad, N., & Johnston, L. A. (2021). A gyrification analysis approach based on Laplace Beltrami eigenfunction level sets. NeuroImage, 229, 117751
Shishegar, R., Rajapakse, S., & Georgiou-Karistianis, N. (2019). Altered Cortical Morphometry in Pre-manifest Huntington’s Disease: Cross-sectional Data from the IMAGE-HD Study. 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), 2844–2847
Soloveva, M. V., Jamadar, S. D., Hughes, M., Velakoulis, D., Poudel, G., & Georgiou-Karistianis, N. (2020a). Brain compensation during response inhibition in premanifest Huntington’s disease. Brain and Cognition, 141, 105560
Soloveva, M. V., Jamadar, S. D., Velakoulis, D., Poudel, G., & Georgiou-Karistianis, N. (2020b). Brain compensation during visuospatial working memory in premanifest Huntington’s disease. Neuropsychologia, 136, 107262
Spalletta, G., Piras, F., & Gili, T. (2018). Brain Morphometry. Springer
Tabrizi, S. J., Langbehn, D. R., Leavitt, B. R., Roos, R. A. C., Durr, A., Craufurd, D. … Stout, J. C. (2009). Biological and clinical manifestations of Huntington’s disease in the longitudinal TRACK-HD study: cross-sectional analysis of baseline data. The Lancet Neurology, 8(9), 791–801
Tereshchenko, A., Magnotta, V., Epping, E., Mathews, K., Espe-Pfeifer, P., Martin, E. … Nopoulos, P. (2019). Brain structure in juvenile-onset Huntington disease. Neurology, 92(17), e1939–e1947
Thieben, M. J., Duggins, A. J., Good, C. D., Gomes, L., Mahant, N., Richards, F. … Frackowiak, R. S. J. (2002). The distribution of structural neuropathology in pre-clinical Huntington’s disease. Brain, 125(8), 1815–1828
van der Plas, E., Langbehn, D. R., Conrad, A. L., Koscik, T. R., Tereshchenko, A., Epping, E. A. … Nopoulos, P. C. (2019). Abnormal brain development in child and adolescent carriers of mutant huntingtin. Neurology, 93(10), e1021–e1030
Van Essen, D. C. (1997). A tension-based theory of morphogenesis and compact wiring in the central nervous system. Nature, 385(6614), 313–318
Wijeratne, P. A., Johnson, E. B., Eshaghi, A., Aksman, L., Gregory, S., Johnson, H. J. … Georgiou-Karistianis, N. (2020). Robust Markers and Sample Sizes for Multicenter Trials of Huntington Disease. Annals of Neurology, 87(5), 751–762
Acknowledgements
We would like to acknowledge all the participants who contributed to this study, the CHDI Foundation Inc. New York (USA), and the National Health and Medical Research Council (NHMRC) for funding IMAGE-HD. We would also like to thank the Royal Children’s Hospital Murdoch Children’s Research Institute for the use of their MRI scanners.
Funding
This work was supported by the CHDI Foundation Inc. New York (USA) (Grant Number A: 3433) and the National Health and Medical Research Council (NHMRC) (Grant Number: 606650) for funding IMAGE-HD. Alex Fornito was additionally supported by the Sylvia and Charles Viertel Charitable Foundation.
Author information
Authors and Affiliations
Contributions
Author contributions included conception and study design (All authors) statistical analysis (BT), interpretation of results (all authors), drafting the manuscript work or revising it critically for important intellectual content (BT, RS, AF and NGK) and approval of final version to be published and agreement to be accountable for the integrity and accuracy of all aspects of the work (All authors).
Corresponding author
Ethics declarations
Ethical approval
Ethical Approval was provided by the Monash University Human Research Ethics Review Committee (Project ID: 14105).
Consent to participate
This study used data collected for the IMAGE-HD study, as such informed consent had previously been obtained.
Consent to publish
As above.
Conflict of interest
The authors declare no conflicts of interest.
Conflict of interest disclosure
None of the authors have a conflict of interest to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tan, B., Shishegar, R., Fornito, A. et al. Longitudinal mapping of cortical surface changes in Huntington’s Disease. Brain Imaging and Behavior 16, 1381–1391 (2022). https://doi.org/10.1007/s11682-021-00625-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11682-021-00625-2