Abstract
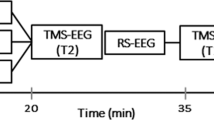
Functional connectivity (FC) is fundamental to brain function and has been implicated in many neuropsychological and neuropsychiatric disorders. It is then of great scientific and clinical interest to find a non-invasive approach to modulate FC. Transcranial magnetic stimulation (TMS) is a non-invasive neuromodulational tool that can affect the target region and remote brain areas. While the distributed effects of TMS are postulated to be through either structural or functional connectivity, an understudied but of great scientific interest question is whether TMS can change the FC between these regions. The purpose of this study was to address this question in normal healthy brain using TMS with continuous theta burst stimulation (cTBS) pulses, which are known to have long-lasting inhibition function. FC was calculated from resting state fMRI before and after real and control (SHAM) stimulation. Compared to SHAM, the repetitive TMS (rTMS) reduces FC between the cTBS target: the left dorsolateral prefrontal cortex (lDLPFC) and brain regions within the default mode network (DMN), proving the effects of rTMS on FC. The reduction of FC might be the results of the inhibitory effects of cTBS rTMS.




Similar content being viewed by others
References
Barker, A. T., Jalinous, R., & Freeston, I. L. (1985). Non-invasive magnetic stimulation of human motor cortex. The Lancet, 325(8437), 1106–1107.
Biswal, B. B., Van Kylen, J., & Hyde, J. S. (1997). Simultaneous assessment of flow and BOLD signals in resting-state functional connectivity maps. NMR in Biomedicine, 10(4–5), 165–170.
Biswal, B., Yetkin, F. Z., Haughton, V. M., & Hyde, J. S. (1995). Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magnetic Resonance in Medicine, 34(4), 537–541.
Bolognini, N., & Ro, T. (2010). Transcranial magnetic stimulation: Disrupting neural activity to Alter and Assess brain function. Journal of Neuroscience, 30(29), 9647–9650.
Chan, R. W., Leong, A. T. L., Ho, L. C., Gao, P. P., Wong, E. C., Dong, C. M., et al. (2017). Low-frequency hippocampal-cortical activity drives brain-wide resting-state functional MRI connectivity. Proceedings of the National Academy of Sciences, 201703309.
Chen, A. C., Oathes, D. J., Chang, C., Bradley, T., Zhou, Z.-W., Williams, L. M., Glover, G. H., Deisseroth, K., & Etkin, A. (2013). Causal interactions between fronto-parietal central executive and default-mode networks in humans. Proceedings of the National Academy of Sciences, 110(49), 19944–19949.
Cordes, D., Haughton, V., Carew, J. D., Arfanakis, K., & Maravilla, K. (2002). Hierarchical clustering to measure connectivity in fMRI resting-state data. Magnetic Resonance Imaging, 20(4), 305–317.
Cordes, D., Haughton, V. M., Arfanakis, K., Wendt, G. J., Turski, P. A., Moritz, C. H., et al. (2000). Mapping functionally related regions of brain with functional connectivity MR imaging. American Journal of Neuroradiology, 21(9), 1636–1644.
Curtis, C. E., & D’Esposito, M. (2003). Persistent activity in the prefrontal cortex during working memory. Trends in Cognitive Sciences, 7(9), 415–423.
Damoiseaux, J. S., Rombouts, S. A. R. B., Barkhof, F., Scheltens, P., Stam, C. J., Smith, S. M., & Beckmann, C. F. (2006). Consistent resting-state networks across healthy subjects. Proceedings of the National Academy of Sciences, 103(37), 13848–13853.
De Luca, M., Smith, S., De Stefano, N., Federico, A., & Matthews, P. M. (2005). Blood oxygenation level dependent contrast resting state networks are relevant to functional activity in the neocortical sensorimotor system. Experimental Brain Research, 167(4), 587–594.
Donghui Song, D. C., Zhang, J., Ge, Q., Zang, Y.-F., & Ze, W. (2018). Associations of brain entropy (BEN) to cerebral blood flow and fractional amplitude of low-frequency fluctuations in the resting brain. Brain imaging and behavior.
Eldaief, M. C., Halko, M. A., Buckner, R. L., & Pascual-Leone, A. (2011). Transcranial magnetic stimulation modulates the brain’s intrinsic activity in a frequency-dependent manner. Proceedings of the National Academy of Sciences, 108(52), 21229–21234.
Fan, J., McCandliss, B. D., Fossella, J., Flombaum, J. I., & Posner, M. I. (2005). The activation of attentional networks. NeuroImage, 26(2), 471–479.
Feredoes, E., Heinen, K., Weiskopf, N., Ruff, C., & Driver, J. (2011). Causal evidence for frontal involvement in memory target maintenance by posterior brain areas during distracter interference of visual working memory. Proceedings of the National Academy of Sciences, 108(42), 17510–17515.
Fitzgerald. (2011). The effects of repetitive transcranial magnetic stimulation in the treatment of depression. Expert Review of Medical Devices, 8(1), 85–95.
Fitzgerald, P. B., Fountain, S., & Daskalakis, Z. J. (2006). A comprehensive review of the effects of rTMS on motor cortical excitability and inhibition. Clinical Neurophysiology, 117(12), 2584–2596.
Fox, M. D., Buckner, R. L., White, M. P., Greicius, M. D., & Pascual-Leone, A. (2012a). Efficacy of transcranial magnetic stimulation targets for depression is related to intrinsic functional connectivity with the subgenual cingulate. Biological Psychiatry, 72(7), 595–603.
Fox, M. D., & Raichle, M. E. (2007). Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nature Reviews Neuroscience, 8(9), 700–711.
Fox, M., Halko, M., Eldaief, M., & Pascual-Leone, A. (2012b). Measuring and manipulating brain connectivity with resting state functional connectivity magnetic resonance imaging (fcMRI) and transcranial magnetic stimulation. Neuroimage, 62(4), 2232–2243.
Fregni, F., & Pascual-Leone, A. (2007). Technology insight: Noninvasive brain stimulation in neurology - perspectives on the therapeutic potential of rTMS and tDCS. Nature Clinical Practice Neurology, 3(7), 383–393.
Friston, K. J. (1994). Functional and effective connectivity in neuroimaging:A synthesis. Human Brain Mapping, 2(2), 56–78.
Friston, K. J., Williams, S., Howard, R., & Frackowiak, R. S. J. (1996). Movement-Related Effects in fMRI Time-Series.
Gratton, T. G. L. (2013). E. M. N. and M. D. Modelling Complex Networks: Cameo Graphs And Transport Processes, 7(Dec), 1–14.
Gratton, C., Lee, T. G., Nomura, E. M., & D’Esposito, M. (2014). Perfusion MRI indexes variability in the functional brain effects of theta-burst transcranial magnetic stimulation. PLoS One, 9(7).
Greicius, M. D. (2008). Resting-state functional connectivity in neuropsychiatric disorders. Current Opinion in Neurology, 21(4), 424–430.
Greicius, M. D., Krasnow, B., Reiss, A. L., & Menon, V. (2003). Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proceedings of the National Academy of Sciences, 100(1), 253–258.
Heekeren, H. R., Marrett, S., Ruff, D. A., Bandettini, P. A., & Ungerleider, L. G. (2006). Involvement of human left dorsolateral prefrontal cortex in perceptual decision making is independent of response modality. Proceedings of the National Academy of Sciences, 103(26), 10023–10028.
Huang, Y. Z., Edwards, M. J., Rounis, E., Bhatia, K. P., & Rothwell, J. C. (2005). Theta burst stimulation of the human motor cortex. Neuron, 45(2), 201–206.
Kobayashi, M., & Pascual-Leone, A. (2003). Transcranial magnetic stimulation in neurology. The Lancet Neurology, 2(3), 145–156.
Lowe, M. J., Dzemidzic, M., Lurito, J. T., Mathews, V. P., & Phillips, M. D. (2000). Correlations in low-frequency BOLD fluctuations reflect cortico-cortical connections. NeuroImage, 12(5), 582–587.
Lowe, M. J., Mock, B. J., & Sorenson, J. A. (1998). Functional connectivity in single and multislice echoplanar imaging using resting-state fluctuations. NeuroImage, 7(2), 119–132.
Maeda, F., Keenan, J. P., Tormos, J. M., Topka, H., & Pascual-Leone, A. (2000). Modulation of corticospinal excitability by repetitive transcranial magnetic stimulation. Clinical Neurophysiology, 111, 800–805.
Mylius, V., Ayache, S. S., Ahdab, R., Farhat, W. H., Zouari, H. G., Belke, M., Brugières, P., Wehrmann, E., Krakow, K., Timmesfeld, N., Schmidt, S., Oertel, W. H., Knake, S., & Lefaucheur, J. P. (2013). Definition of DLPFC and M1 according to anatomical landmarks for navigated brain stimulation: Inter-rater reliability, accuracy, and influence of gender and age. NeuroImage, 78, 224–232.
Oberman, L., Edwards, D., Eldaief, M., & Pascual-Leone, A. (2011). Safety of theta burst transcranial magnetic stimulation: A systematic review of the literature. Journal of Clinical Neurophysiology, 28(1), 67–74.
Orosz, A., Jann, K., Wirth, M., Wiest, R., Dierks, T., & Federspiel, A. (2012). Theta burst TMS increases cerebral blood flow in the primary motor cortex during motor performance as assessed by arterial spin labeling (ASL). NeuroImage, 61(3), 599–605.
Raichle, M. E., MacLeod, A. M., Snyder, A. Z., Powers, W. J., Gusnard, D. A., & Shulman, G. L. (2001). A default mode of brain function. Proceedings of the National Academy of Sciences, 98(2), 676–682.
Rossi, S., Hallett, M., Rossini, P. M., Pascual-Leone, A., Avanzini, G., Bestmann, S., et al. (2009). Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clinical Neurophysiology, 120(12), 2008–2039.
Rossini, P. M., & Rossi, S. (2007). Transcranial magnetic stimulation: Diagnostic, therapeutic, and research potential. Neurology, 68(7), 484–488.
Ruff, C. C., Driver, J., & Bestmann, S. (2009). Combining TMS and fMRI: From “virtual lesions” to functional-network accounts of cognition. Cortex, 45(9), 1043–1049.
Shang, Y.-Q., Xie, J., Peng, W., Zhang, J., Chang, D., & Wang, Z. (2018). Network-wise cerebral blood flow redistribution after 20 Hz rTMS on left dorso-lateral prefrontal cortex. European Journal of Radiology, 101, 144–148.
Siebner, H. R., Bergmann, T. O., Bestmann, S., Massimini, M., Johansen-Berg, H., Mochizuki, H., Bohning, D. E., Boorman, E. D., Groppa, S., Miniussi, C., Pascual-Leone, A., Huber, R., Taylor, P. C. J., Ilmoniemi, R. J., de Gennaro, L., Strafella, A. P., Kähkönen, S., Klöppel, S., Frisoni, G. B., George, M. S., Hallett, M., Brandt, S. A., Rushworth, M. F., Ziemann, U., Rothwell, J. C., Ward, N., Cohen, L. G., Baudewig, J., Paus, T., Ugawa, Y., & Rossini, P. M. (2009). Consensus paper: Combining transcranial stimulation with neuroimaging. Brain Stimulation, 2(2), 58–80.
Smittenaar, P., FitzGerald, T. H. B., Romei, V., Wright, N. D., & Dolan, R. J. (2013). Disruption of dorsolateral prefrontal cortex decreases model-based in favor of model-free control in humans. Neuron, 80(4), 914–919.
Song, D., Chang, D., Zhang, J., Peng, W., Shang, Y., Gao, X., & Wang, Z. (2018). Reduced brain entropy by repetitive transcranial magnetic stimulation on the left dorsolateral prefrontal cortex in healthy young adults. Brain Imaging and Behavior.
Valero-Cabré, A., Payne, B. R., & Pascual-Leone, A. (2007). Opposite impact on 14C-2-deoxyglucose brain metabolism following patterns of high and low frequency repetitive transcranial magnetic stimulation in the posterior parietal cortex. Experimental Brain Research, 176(4), 603–615.
Valero-Cabré, A., Payne, B. R., Rushmore, J., Lomber, S. G., & Pascual-Leone, A. (2005). Impact of repetitive transcranial magnetic stimulation of the parietal cortex on metabolic brain activity: A 14C-2DG tracing study in the cat. Experimental Brain Research, 163(1), 1–12.
van den Heuvel, M., Mandl, R., & Pol, H. H. (2008). Normalized cut group clustering of resting-state fMRI data. PLoS One, 3(4).
Verdon, C.M., Saba, G. & Januel, D., (2004). Stimulation magnétique transcrânienne et fonctions cognitives. L'Encéphale, 30(4), 363–368.
Wagner, T., Valero-Cabre, A., & Pascual-Leone, A. (2007). Noninvasive human brain stimulation. Annual Review of Biomedical Engineering, 9(1), 527–565.
Wang, J. X., Rogers, L. M., Gross, E. Z., Ryals, A. J., Dokucu, M. E., Brandstatt, K. L., . . . Voss, J. L. (2014b). Targeted enhancement of cortical-hippocampal brain networks and associative memory Jane X. Wang, 1054.
Wang, Z., Aguirre, G. K., Rao, H., Wang, J., Fernández-Seara, M. A., Childress, A. R., & Detre, J. A. (2008). Empirical optimization of ASL data analysis using an ASL data processing toolbox: ASLtbx. Magnetic Resonance Imaging, 26(2), 261–269.
Wang, Z., Li, Y., Childress, A. R., & Detre, J. A. (2014a). Brain entropy mapping using fMRI. PLoS One, 9(3), 1–8.
Wassermann, E. M., & Lisanby, S. H. (2001). Therapeutic application of repetitive transcranial magnetic stimulation: A review. Clinical Neurophysiology : Official Journal of the International Federation of Clinical Neurophysiology, 112, 1367–1377.
Xiong, J. H., Parsons, L. M., Gao, J. H., & Fox, P. T. (1999). Interregional connectivity to primary motor cortex revealed using MRI resting state images. Human Brain Mapping, 8(2–3), 151–156.
Xue, S. W., Guo, Y., Peng, W., Zhang, J., Chang, D., Zang, Y. F., & Wang, Z. (2017). Increased low-frequency resting-state brain activity by high-frequency repetitive TMS on the left dorsolateral prefrontal cortex. Frontiers in Psychology, 8(Dec), 1–8.
Yan, C.-G., & Zang, Y.-F. (2010). DPARSF: A MATLAB toolbox for “pipeline” data analysis of resting-state fMRI. Frontiers in System Neuroscience, 4(May), 1–7.
Zhang, D., & Raichle, M. E. (2010). Disease and the brain’s dark energy. Nature Reviews Neurology, 6(1), 15–28.
Acknowledgements
This study was supported by National Natural Science Foundation of China (No. 61671198), the Youth 1000 Talent Program of China.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declared no conflict of interest regarding the study reported in this paper.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animalsperformed by any of the authors.
Informed consent
Informed written consents were obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shang, Y., Chang, D., Zhang, J. et al. Theta-burst transcranial magnetic stimulation induced functional connectivity changes between dorsolateral prefrontal cortex and default-mode-network. Brain Imaging and Behavior 14, 1955–1963 (2020). https://doi.org/10.1007/s11682-019-00139-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11682-019-00139-y