Abstract
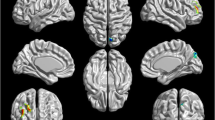
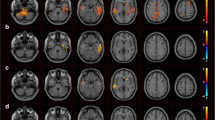
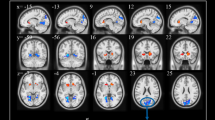
Several resting-state neuroimaging studies have indicated abnormal regional homogeneity (ReHo) in chronic schizophrenia; however, little work has been conducted to investigate naïve patients with first-episode schizophrenia (FES). Even less investigated is the association between ReHo measures and clinical symptom severity in naïve patients with FES. The current study evaluated ReHo alterations in whole brain, and assessed the correlations between ReHo measures and clinical variables in naïve patients with FES. Forty-four naïve patients with FES and 26 healthy controls (HC) underwent resting-state functional magnetic resonance imaging (rs-fMRI). Group-level analysis was utilized to analyze the ReHo differences between FES and HC in a voxel-by-voxel manner. Severity of symptoms was evaluated using a five-factor model of the Positive and Negative Syndrome Scale (PANSS). The correlation between the severity of symptoms and ReHo map was examined in patients using voxel-wise correlation analyses within brain areas that showed a significant ReHo alteration in patients compared with controls. Compared with the healthy control group, the FES group showed a significant decrease in ReHo values in the left medial frontal gyrus (MFG), right precentral gyrus, left superior temporal gyrus (STG), left left middle temporal gyrus (MTG), left thalamus, and significant increase in ReHo values in the left MFG, left inferior parietal lobule (IPL), left precuneus, and right lentiform nucleus (LN). In addition, the correlation analysis showed the PANSS total score negatively correlated with ReHo in the right precentral gyrus and positively correlated with ReHo in the left thalamus, the positive factor positively correlated with ReHo in the right thalamus, the disorganized/concrete factor positively correlated with ReHo in left posterior cingulate gyrus (PCG), the excited factor positively correlated with ReHo in the left precuneus, and the depressed factor negatively correlated with ReHo in the right postcentral gyrus and positively correlated with ReHo in the right thalamus. Our results indicate that widespread ReHo abnormalities occurred in an early stage of schizophrenic onset, suggesting a potential neural basis for the pathogenesis and symptomatology of schizophrenia.


Similar content being viewed by others
References
Andreasen, N. C., et al. (1994). Thalamic abnormalities in schizophrenia visualized through magnetic resonance image averaging. Science, 266(5183), 294–298.
Andreasen, N. C., Paradiso, S., & O'Leary, D. S. (1998). "Cognitive dysmetria" as an integrative theory of schizophrenia: a dysfunction in cortical-subcortical-cerebellar circuitry? Schizophrenia Bulletin, 24(2), 203–218.
Ashburner, J. (2007). A fast diffeomorphic image registration algorithm. NeuroImage, 38(1), 95–113.
Biswal, B., Zerrin Yetkin, F., Haughton, V. M., & Hyde, J. S. (1995). Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magnetic Resonance in Medicine, 34(4), 537–541.
Boutros, N. N., Arfken, C., Galderisi, S., Warrick, J., Pratt, G., & Iacono, W. (2008). The status of spectral EEG abnormality as a diagnostic test for schizophrenia. Schizophrenia Research, 99(1–3), 225–237.
Buckley, P. E., Evans, D.. (2006). First-episode schizophrenia. A window of opportunity for optimizing care and outcomes. Postgrad Med Spec No: p. 5–19.
Catafau, A. M., Parellada, E., Lomeña, F. J., Bernardo, M., Pavía, J., Ros, D., Setoain, J., & Gonzalez-Monclús, E. (1994). Prefrontal and temporal blood flow in schizophrenia: resting and activation technetium-99m-HMPAO SPECT patterns in young neuroleptic-naive patients with acute disease. Journal of Nuclear Medicine, 35(6), 935–941.
Cavanna, A. E., & Trimble, M. R. (2006). The precuneus: a review of its functional anatomy and behavioural correlates. Brain, 129(Pt 3), 564–583.
Chao-Gan, Y., & Yu-Feng, Z. (2010). DPARSF: A MATLAB toolbox for "pipeline" data analysis of resting-state fMRI. Frontiers in Systems Neuroscience, 4, 13.
Chen, J., et al. (2013). Comparative study of regional homogeneity in schizophrenia and major depressive disorder. American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics, 162B(1), 36–43.
Cui, L. B., Liu, K., Li, C., Wang, L. X., Guo, F., Tian, P., Wu, Y. J., Guo, L., Liu, W. M., Xi, Y. B., Wang, H. N., & Yin, H. (2016). Putamen-related regional and network functional deficits in first-episode schizophrenia with auditory verbal hallucinations. Schizophrenia Research, 173(1–2), 13–22.
Danckert, J., Saoud, M., & Maruff, P. (2004). Attention, motor control and motor imagery in schizophrenia: implications for the role of the parietal cortex. Schizophrenia Research, 70(2–3), 241–261.
Ebmeier, K., et al. (1993). Single-photon emission computed tomography with 99mTc-exametazime in unmediated schizophrenic patients. Biological Psychiatry, 33(7), 487–495.
Fair, D. A., Cohen, A. L., Dosenbach, N. U. F., Church, J. A., Miezin, F. M., Barch, D. M., Raichle, M. E., Petersen, S. E., & Schlaggar, B. L. (2008). The maturing architecture of the brain's default network. Proceedings of the National Academy of Sciences of the United States of America, 105(10), 4028–4032.
Fang, L. (2013). Resting-state functional magnetic resonance imaging study of brain function in the first-episode paranoid-type schizophrenia patients. Nanjing: The Fourth School of Clinical Medicine, Nanjing Medical University.
Friston, K. J., Williams, S., Howard, R., Frackowiak, R. S. J., & Turner, R. (1996). Movement-related effects in fMRI time-series. Magnetic Resonance in Medicine, 35(3), 346–355.
Gao, B., Wang, Y., Liu, W., Chen, Z., Zhou, H., Yang, J., Cohen, Z., Zhu, Y., & Zang, Y. (2015). Spontaneous activity associated with delusions of schizophrenia in the left medial superior frontal gyrus: A resting-state fMRI study. PLoS One, 10(7), e0133766.
Garrity, A. G., Pearlson, G. D., McKiernan, K., Lloyd, D., Kiehl, K. A., & Calhoun, V. D. (2007). Aberrant "default mode" functional connectivity in schizophrenia. The American Journal of Psychiatry, 164(3), 450–457.
Greicius, M. D., Krasnow, B., Reiss, A. L., & Menon, V. (2003). Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proceedings of the National Academy of Sciences of the United States of America, 100(1), 253–258.
Harrison, B. J., Yücel, M., Pujol, J., & Pantelis, C. (2007). Task-induced deactivation of midline cortical regions in schizophrenia assessed with fMRI. Schizophrenia Research, 91(1–3), 82–86.
Hu, M. L., et al. (2016). Short-term effects of risperidone monotherapy on spontaneous brain activity in first-episode treatment-naive schizophrenia patients: a longitudinal fMRI study. Scientific Reports, 6, 34287.
Hu, M. L., Zong, X. F., Mann, J. J., Zheng, J. J., Liao, Y. H., Li, Z. C., He, Y., Chen, X. G., & Tang, J. S. (2017). A review of the functional and anatomical default mode network in schizophrenia. Neuroscience Bulletin, 33(1), 73–84.
Jani, M., & Kasparek, T. (2017). Emotion recognition and theory of mind in schizophrenia: a meta-analysis of neuroimaging studies. The World Journal of Biological Psychiatry, 1–31.
Jardri, R., Thomas, P., Delmaire, C., Delion, P., & Pins, D. (2013). The neurodynamic organization of modality-dependent hallucinations. Cerebral Cortex, 23(5), 1108–1117.
Jerrell, J. M., & Hrisko, S. (2013a). A comparison of the PANSS pentagonal and van Der Gaag 5-factor models for assessing change over time. Psychiatry Research, 207(1–2), 134–139.
Jerrell, J. M., & Hrisko, S. (2013b). Utility of two PANSS 5-factor models for assessing psychosocial outcomes in clinical programs for persons with schizophrenia. Schizophr Res Treatment, 2013, 705631.
Jiang, L., & Zuo, X. N. (2016). Regional homogeneity: a multimodal, multiscale neuroimaging marker of the human connectome. The Neuroscientist, 22(5), 486–505.
Jiang, S., Zhou, B., & Liao, Y. (2010). Primary study of resting state functional magnetic resonance imaging in early onset schizophrenia using ReHo. Zhong Nan Da Xue Xue Bao. Yi Xue Ban, 35(9), 947–951.
Jiang, L., Xu, T., He, Y., Hou, X. H., Wang, J., Cao, X. Y., Wei, G. X., Yang, Z., He, Y., & Zuo, X. N. (2015a). Toward neurobiological characterization of functional homogeneity in the human cortex: regional variation, morphological association and functional covariance network organization. Brain Structure & Function, 220(5), 2485–2507.
Jiang, L., Xu, Y., Zhu, X. T., Yang, Z., Li, H. J., & Zuo, X. N. (2015b). Local-to-remote cortical connectivity in early- and adulthood-onset schizophrenia. Translational Psychiatry, 5, e566.
Kaplan, R. D., Szechtman, H., Franco, S., Szechtman, B., Nahmias, C., Garnett, E. S., List, S., & Cleghorn, J. M. (1993). Three clinical syndromes of schizophrenia in untreated subjects relation to brain glucose activity measured by position emission tomography (PET). Schizophrenia Research, 11(1), 47–54.
Katanoda, K., Matsuda, Y., & Sugishita, M. (2002). A spatio-temporal regression model for the analysis of functional MRI data. NeuroImage, 17(3), 1415–1428.
Kay, S. R., Fiszbein, A., & Opler, L. A. (1987). The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophrenia Bulletin, 13(2), 261–276.
Kay, S. R., Opler, L. A., & Lindenmayer, J. P. (1988). Reliability and validity of the positive and negative syndrome scale for schizophrenics. Psychiatry Research, 23(1), 99–110.
Kim, G. W., Kim, Y. H., & Jeong, G. W. (2017). Whole brain volume changes and its correlation with clinical symptom severity in patients with schizophrenia: A DARTEL-based VBM study. PLoS One, 12(5), e0177251.
Knott, V., Labelle, A., Jones, B., & Mahoney, C. (2001). Quantitative EEG in schizophrenia and in response to acute and chronic clozapine treatment. Schizophrenia Research, 50(1–2), 41–53.
Kumari, V., Peters, E., Guinn, A., Fannon, D., Russell, T., Sumich, A., Kuipers, E., Williams, S. C. R., & ffytche, D. H. (2016). Mapping depression in schizophrenia: a functional magnetic resonance imaging study. Schizophrenia Bulletin, 42(3), 802–813.
Lancon, C., et al. (2000). Stability of the five-factor structure of the positive and negative syndrome scale (PANSS). Schizophrenia Research, 42(3), 231–239.
Lee, S. H., Wynn, J. K., Green, M. F., Kim, H., Lee, K. J., Nam, M., Park, J. K., & Chung, Y. C. (2006). Quantitative EEG and low resolution electromagnetic tomography (LORETA) imaging of patients with persistent auditory hallucinations. Schizophrenia Research, 83(2–3), 111–119.
Levitt, J. J., et al. (2010). A selective review of volumetric and morphometric imaging in schizophrenia. Current Topics in Behavioral Neurosciences, 4, 243–281.
Levy, A. V., Gomez-Mont, F., Volkow, N. D., Corona, J. F., Brodie, J. D., & Cancro, R. (1992). Spatial low frequency pattern analysis in positron emission tomography: a study between normals and schizophrenics. Journal of Nuclear Medicine, 33(2), 287–295.
Li, H. J., Xu, Y., Zhang, K. R., Hoptman, M. J., & Zuo, X. N. (2015). Homotopic connectivity in drug-naive, first-episode, early-onset schizophrenia. Journal of Child Psychology and Psychiatry, 56(4), 432–443.
Liao, H., Wang, L., Zhou, B., Tang, J., Tan, L., Zhu, X., Yi, J., Chen, X., & Tan, C. (2012). A resting-state functional magnetic resonance imaging study on the first-degree relatives of persons with schizophrenia. Brain Imaging and Behavior, 6(3), 397–403.
Lindenmayer, J. P., Bossie, C. A., Kujawa, M., Zhu, Y., & Canuso, C. M. (2008). Dimensions of psychosis in patients with bipolar mania as measured by the positive and negative syndrome scale. Psychopathology, 41(4), 264–270.
Liu, H., Liu, Z., Liang, M., Hao, Y., Tan, L., Kuang, F., Yi, Y., Xu, L., & Jiang, T. (2006). Decreased regional homogeneity in schizophrenia: a resting state functional magnetic resonance imaging study. Neuroreport, 17(1), 19–22.
Liu, C., Xue, Z., Palaniyappan, L., Zhou, L., Liu, H., Qi, C., Wu, G., Mwansisya, T. E., Tao, H., Chen, X., Huang, X., Liu, Z., & Pu, W. (2016). Abnormally increased and incoherent resting-state activity is shared between patients with schizophrenia and their unaffected siblings. Schizophrenia Research, 171(1–3), 158–165.
Lui, S., Li, T., Deng, W., Jiang, L., Wu, Q., Tang, H., Yue, Q., Huang, X., Chan, R. C., Collier, D. A., Meda, S. A., Pearlson, G., Mechelli, A., Sweeney, J. A., & Gong, Q. (2010). Short-term effects of antipsychotic treatment on cerebral function in drug-naive first-episode schizophrenia revealed by "resting state" functional magnetic resonance imaging. Archives of General Psychiatry, 67(8), 783–792.
Malaspina, D., Harkavy-Friedman, J., Corcoran, C., Mujica-Parodi, L., Printz, D., Gorman, J. M., & van Heertum, R. (2004). Resting neural activity distinguishes subgroups of schizophrenia patients. Biological Psychiatry, 56(12), 931–937.
Maruff, P., et al. (2005). Reduced volume of parietal and frontal association areas in patients with schizophrenia characterized by passivity delusions. Psychological Medicine, 35(6), 783–789.
Meng, X., et al. (2017). Predicting individualized clinical measures by a generalized prediction framework and multimodal fusion of MRI data. NeuroImage, 145, 218–229.
Mondino, M., Jardri, R., Suaud-Chagny, M. F., Saoud, M., Poulet, E., & Brunelin, J. (2016). Effects of Fronto-temporal transcranial direct current stimulation on auditory verbal hallucinations and resting-state functional connectivity of the left Temporo-parietal junction in patients with schizophrenia. Schizophrenia Bulletin, 42(2), 318–326.
Mukherjee, P., Whalley, H. C., McKirdy, J. W., McIntosh, A. M., Johnstone, E. C., Lawrie, S. M., & Hall, J. (2012). Lower effective connectivity between amygdala and parietal regions in response to fearful faces in schizophrenia. Schizophrenia Research, 134(2–3), 118–124.
Obeso, J. A., Rodriguez-Oroz, M. C., Stamelou, M., Bhatia, K. P., & Burn, D. J. (2014). The expanding universe of disorders of the basal ganglia. The Lancet, 384(9942), 523–531.
Ohtani, T., Levitt, J. J., Nestor, P. G., Kawashima, T., Asami, T., Shenton, M. E., Niznikiewicz, M., & McCarley, R. W. (2014). Prefrontal cortex volume deficit in schizophrenia: a new look using 3T MRI with manual parcellation. Schizophrenia Research, 152(1), 184–190.
Pardo, B. M., Garolera, M., Ariza, M., Pareto, D., Salamero, M., Valles, V., Delgado, L., & Alberni, J. (2011). Improvement of cognitive flexibility and cingulate blood flow correlates after atypical antipsychotic treatment in drug-naive patients with first-episode schizophrenia. Psychiatry Research, 194(3), 205–211.
Parellada, E., Catafau, A. M., Bernardo, M., Lomeña, F., González-Monclús, E., & Setoain, J. (1994). Prefrontal dysfunction in young acute neuroleptic-naive schizophrenic patients: A resting and activation SPECT study. Psychiatry Research: Neuroimaging, 55(3), 131–139.
Pascual-Marqui, R. D., Lehmann, D., Koenig, T., Kochi, K., Merlo, M. C. G., Hell, D., & Koukkou, M. (1999). Low resolution brain electromagnetic tomography (LORETA) functional imaging in acute, neuroleptic-naive, first-episode, productive schizophrenia. Psychiatry Research, 90(3), 169–179.
Power, J. D., Barnes, K. A., Snyder, A. Z., Schlaggar, B. L., & Petersen, S. E. (2012). Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage, 59(3), 2142–2154.
Premkumar, P., Fannon, D., Kuipers, E., Cooke, M. A., Simmons, A., & Kumari, V. (2008). Association between a longer duration of illness, age and lower frontal lobe grey matter volume in schizophrenia. Behavioural Brain Research, 193(1), 132–139.
Raichle, M. E., & Gusnard, D. A. (2005). Intrinsic brain activity sets the stage for expression of motivated behavior. The Journal of Comparative Neurology, 493(1), 167–176.
Roth, R. M., Flashman, L. A., Saykin, A. J., McAllister, T. W., & Vidaver, R. (2004). Apathy in schizophrenia: reduced frontal lobe volume and neuropsychological deficits. The American Journal of Psychiatry, 161(1), 157–159.
Semkovska, M., Bedard, M. A., & Stip, E. (2001). Hypofrontality and negative symptoms in schizophrenia: synthesis of anatomic and neuropsychological knowledge and ecological perspectives. Encephale, 27(5), 405–415.
Shenton, M. E., Kikinis, R., Jolesz, F. A., Pollak, S. D., LeMay, M., Wible, C. G., Hokama, H., Martin, J., Metcalf, D., Coleman, M., & McCarley, R. W. (1992). Abnormalities of the left temporal lobe and thought disorder in schizophrenia. A quantitative magnetic resonance imaging study. The New England Journal of Medicine, 327(9), 604–612.
Simpson, E. H., Kellendonk, C., & Kandel, E. (2010). A possible role for the striatum in the pathogenesis of the cognitive symptoms of schizophrenia. Neuron, 65(5), 585–596.
Smith, S. M., & Nichols, T. E. (2009). Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. NeuroImage, 44(1), 83–98.
Tandon, R., Nasrallah, H. A., & Keshavan, M. S. (2010). Schizophrenia, "just the facts" 5. Treatment and prevention. Past, present, and future. Schizophrenia Research, 122(1–3), 1–23.
Taylor, S. F., Kang, J., Brege, I. S., Tso, I. F., Hosanagar, A., & Johnson, T. D. (2012). Meta-analysis of functional neuroimaging studies of emotion perception and experience in schizophrenia. Biological Psychiatry, 71(2), 136–145.
Tononi, G., McIntosh, A. R., Russell, D. P., & Edelman, G. M. (1998). Functional clustering: identifying strongly interactive brain regions in neuroimaging data. NeuroImage, 7(2), 133–149.
Tseng, H. H., Chen, S. H., Liu, C. M., Howes, O., Huang, Y. L., Hsieh, M. H., Liu, C. C., Shan, J. C., Lin, Y. T., & Hwu, H. G. (2013). Facial and prosodic emotion recognition deficits associate with specific clusters of psychotic symptoms in schizophrenia. PLoS One, 8(6), e66571.
Wallwork, R. S., Fortgang, R., Hashimoto, R., Weinberger, D. R., & Dickinson, D. (2012). Searching for a consensus five-factor model of the positive and negative syndrome scale for schizophrenia. Schizophrenia Research, 137(1–3), 246–250.
Watanuki, T., Matsuo, K., Egashira, K., Nakashima, M., Harada, K., Nakano, M., Matsubara, T., Takahashi, K., & Watanabe, Y. (2016). Precentral and inferior prefrontal hypoactivation during facial emotion recognition in patients with schizophrenia: A functional near-infrared spectroscopy study. Schizophrenia Research, 170(1), 109–114.
Whitfield-Gabrieli, S., & Ford, J. M. (2012). Default mode network activity and connectivity in psychopathology. Annual Review of Clinical Psychology, 8, 49–76.
Whitfield-Gabrieli, S., Thermenos, H. W., Milanovic, S., Tsuang, M. T., Faraone, S. V., McCarley, R. W., Shenton, M. E., Green, A. I., Nieto-Castanon, A., LaViolette, P., Wojcik, J., Gabrieli, J. D. E., & Seidman, L. J. (2009). Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proceedings of the National Academy of Sciences of the United States of America, 106(4), 1279–1284.
Xiao, B., Wang, S., Liu, J., Meng, T., He, Y., & Luo, X. (2017). Abnormalities of localized connectivity in schizophrenia patients and their unaffected relatives: a meta-analysis of resting-state functional magnetic resonance imaging studies. Neuropsychiatric Disease and Treatment, 13, 467–475.
Xiong, Y. (2016). Resting state fMRI study of amplitude of low-frequency fluctuation and regional homogeneity in early onset schizophrenia. Taiyuan: First Hospital, Sanxi Medical University.
Yan, C. G., Wang, X. D., Zuo, X. N., & Zang, Y. F. (2016). DPABI: data processing & analysis for (resting-state) brain imaging. Neuroinformatics, 14(3), 339–351.
Yang, Z., et al. (2014). Brain network informed subject community detection in early-onset schizophrenia. Scientific Reports, 4, 5549.
Yu, R., Hsieh, M. H., Wang, H. L. S., Liu, C. M., Liu, C. C., Hwang, T. J., Chien, Y. L., Hwu, H. G., & Tseng, W. Y. I. (2013). Frequency dependent alterations in regional homogeneity of baseline brain activity in schizophrenia. PLoS One, 8(3), e57516.
Zang, Y., Jiang, T., Lu, Y., He, Y., & Tian, L. (2004). Regional homogeneity approach to fMRI data analysis. NeuroImage, 22(1), 394–400.
Zhou, Y., Liang, M., Tian, L., Wang, K., Hao, Y., Liu, H., Liu, Z., & Jiang, T. (2007). Functional disintegration in paranoid schizophrenia using resting-state fMRI. Schizophrenia Research, 97(1–3), 194–205.
Zuo, X. N., & Xing, X. X. (2014). Test-retest reliabilities of resting-state FMRI measurements in human brain functional connectomics: a systems neuroscience perspective. Neuroscience and Biobehavioral Reviews, 45, 100–118.
Zuo, X. N., Xu, T., Jiang, L., Yang, Z., Cao, X. Y., He, Y., Zang, Y. F., Castellanos, F. X., & Milham, M. P. (2013). Toward reliable characterization of functional homogeneity in the human brain: preprocessing, scan duration, imaging resolution and computational space. NeuroImage, 65, 374–386.
Acknowledgements
This work is partly supported by the Natural Science Foundation of Jiangsu Province (Grants No BK20151076) and the project of Jiangsu Provincial Health Department (General Program No: H201442,Y2013004), the Six talent peaks project in Jiangsu Province (NO2014-WSN-055).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All research procedures were approved by the Medical Research Ethics Committee of Nanjing Brain Hospital, and were conducted in accordance with the 1964 Helsinki declaration and its later amendments.
Informed consent
Written informed consent of the schizophrenic patient was obtained from his/her legally authorized representative and the control provided written informed consent himself/herself after totally understanding the purpose of our study.
Conflicts of interest
Xiaoxin Zhao, Jingjing Yao, Yiding Lv, Xinyue Zhang, Chongyang Han, Lijun Chen, Fangfang Ren, Zhuma Jin, Yuan Li, and Yuxiu Sui declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Zhao, X., Yao, J., Lv, Y. et al. Abnormalities of regional homogeneity and its correlation with clinical symptoms in Naïve patients with first-episode schizophrenia. Brain Imaging and Behavior 13, 503–513 (2019). https://doi.org/10.1007/s11682-018-9882-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11682-018-9882-4