Abstract
Background
Tears and lesions of the rotator cuff are a frequent cause of shoulder pain and disability. Surgical repair of the rotator cuff is a valuable procedure to improve shoulder function and decrease pain. However, there is no consensus concerning the rehabilitation protocol following surgery.
Objectives
To review and evaluate current rehabilitation contents and protocols after rotator cuff repair by reviewing the existing scientific literature and providing an overview of the clinical practice of selected German Society of Shoulder and Elbow Surgery e. V. (DVSE) shoulder experts.
Materials and methods
A literature search for the years 2004–2014 was conducted in relevant databases and bibliographies including the Guidelines International Network, National Guidelines, PubMed, Cochrane Central
Register of Controlled Trials, Cochrane Database of Systematic Reviews, and the Physiotherapy Evidence Database. In addition, 63 DVSE experts were contacted via online questionnaire.
Results
A total of 17 studies, four reviews and one guideline fulfilled the inclusion criteria. Based on these results and the obtained expert opinions, a four-phase rehabilitation protocol could be developed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tears of the rotator cuff tendons (RC) are a frequent cause of shoulder complaints [44]. Improvement in terms of strength, movement and pain reduction can be expected after rotator cuff repair surgery [27]. Unfortunately, there is no consensus on the rehabilitation protocols and contents following the surgical procedure [24]. Conventional rehabilitation protocols after reconstruction of the rotator cuff (RCR) often vary considerably, even in terms of basic content such as the length of immobilization, movement limitations and whether or not an orthosis should be used. There still is a lack of evidence for many common forms of rehabilitation contents, although in many health care systems evidence-based medicine has gained ground. In Germany, among others, the guideline program of the German Pension Insurance Association focused on this conflict [23, 25].
The rehabilitation commission of the German Society for Shoulder and Elbow Surgery (DVSE) has studied this issue intensively. The aim of this paper was, firstly, to conduct an evidence-based evaluation of the most important forms of treatment after RCR, based on an extensive literature review and, with the help of a survey among DVSE shoulder experts, to determine if there is an existing best-clinical practice consensus for or against specific forms of treatment.
Materials and methods
Literature review
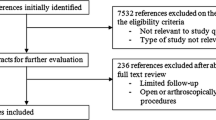
The literature search had a hierarchical structure (best available evidence) based on guidelines, health technology assessments (HTA), systematic reviews and clinical studies that investigated post-operative rehabilitation after RCR. This was supplemented by an analysis of the primary literature under examination (Fig. 1). We started by searching for national and international guidelines in the databases of the “Guidelines International Network” (http://www.g-i-n.net/), various other national guidelines (National Guideline Clearinghouse, AWMF, SIGN, NICE) and HTA (INAHTA, HTAi, EUnetHTA, DIMDI, IQWiG).
A search for meta analyses, systematic reviews and primary studies was conducted using the electronic databases Medline via PubMed, the Cochrane Central Register of Controlled Trials, the Cochrane Database of Systematic Reviews and the Physiotherapy Evidence Database (PEDro). The period between 1/2004 and 10/2014 was the period of reference. In addition, a manual search was conducted that included general internet research, a screening of the literature references listed in the collected articles, and a renewed assessment of various journals. A manual search for relevant animal studies was also performed for the topics of tendon healing and length of immobilization. The relevant publications were selected based on how relevant their content was to the issue, whether they were in English or German, and whether the comparative studies enlisted at least ten patients per group.
The literature was selected based on the PICO concept of the Cochrane Institute (Table 1). The levels of evidence were interpreted based on the classifications of the Oxford Center for Evidence-Based Medicine 2009 (OCEBM).
The PEDro scale (http://www.pedro.org.au) was used to analyze the individual studies and the systematic reviews were assessed according to AMSTAR (assessment of multiple systematic reviews; http://amstar.ca). Only studies that demonstrated the highest obtainable level of evidence served as the basis for the consensus paper. At least two studies were required per topic. For example, if there was only one Level I study done on the topic, Level II studies were also taken into account. If there were two or more Level I studies, no Level II, III and IV studies were considered. Consensus topics that were not presented accordingly in the literature were included on the basis of “best available evidence”.
Expert opinions
After evaluating the literature, the DVSE’s Rehabilitation Commission decided which topics required the opinions of the DVSE experts. The individual topics were assigned to the following groups:
-
1.
Immobilization and arm positioning
-
2.
Physical therapy (cryotherapy, electrotherapy, hydrotherapy)
-
3.
Physiotherapy, self-exercise and CPM (continuous passive motion)
-
4.
Rehabilitation protocols
The online tool Surveymonkey (www.surveymonkey.com) was used to survey 63 selected DVSE experts between 2/2015 and 4/2015. The participation rate was 69.8%.
Results and discussion
The guideline search resulted in one hit for the American Academy of Orthopedic Surgeons. Two systematic reviews and 13 clinical studies in the databases were examined. A manual search revealed two additional systematic reviews and four individual studies. An overview of all the papers is listed in Tables 2, 3 and 4.
In order to do justice to the amount of information contained in each publication, the individual sub-topics of the overall rehabilitation process are thematically assessed and discussed in individual sub-sections below. Furthermore, the results of the expert survey are presented according to topic.
Immobilization and arm positioning
Directly after the operation, the question arises as to whether and to what extent the shoulder should be immobilized. The risk of a re-rupture or disrupted tendon healing as a result of too much strain have to be weighed against a stiff shoulder caused by too little mobilization. Cadaver studies reveal that the so-called “time zero strength” of the sutured supraspinatus tendon resists 70–100% of the forces affecting it [40]. However, biomechanical studies have shown there is a “gapping effect” for cyclical, clinically relevant strain, even in the case of double row suture techniques [40]. As tendon healing progresses, the biomechanical properties of the tendon-suture-construct change. Therefore, the time it takes for tendons to heal should be taken into account. Animal studies are frequently referred to, since the tendon healing process has already been intensively studied in animals. In animal models a fragile scar appears 0–14 days after the operation during the inflammatory phase [7]. In the subsequent proliferative phase, 3–4 weeks after the operation, fibroblasts, myofibroblasts and endothelial cells appear, neoangiogenesis begins, and a stronger tendon-bone connection develops. In the maturation and remodeling phase, starting in weeks 4 to 6, collagen III is increasingly replaced by mature collagen I and the tendon integrates more strongly and stably into the bone.
Animal studies have shown that the time it takes to achieve full strength varies between 12 and 26 months [7]. When the issue of early exercise therapy is translated to animal models, difficulties arise in comparing and interpreting the different animal models. It is also difficult to standardize any exercises for animals. Transferring the findings to humans also poses a challenge.
Li et al. [30] found that early passive exercise benefited tendon healing in rabbits. Peltz et al. [36] demonstrated in a rat model that movement was poorer when there was passive exercise directly after the operation as a result of increased scar formation. There were no differences with respect to tendon healing. By contrast, Gimbel et al. [16] found in rat models that the healing tendon had better mechanical properties when immobilization was extended. However, it is interesting to note that complete strain reduction using a botulinum toxin appears to have negative effects on tendon recovery in animal models [13]. In a comparison study of rabbits that compared immediately allowing movement, short-term immobilization with subsequent passive exercise and complete immobilization [47] Zhang et al. found that direct, post-operative passive exercise with intermittent immobilization did not negatively affect tendon healing histologically and in magnetic resonance imaging (MRI). However, tendon healing was found to diminish when function was completely allowed.
Compared to these heterogeneous animal studies, prospective studies of humans provide a good level of data. Early passive exercise does not appear to be disadvantageous [19]. Both Chan et al. [6] and Shen et al. [41] were able to show in meta analyses of randomized clinical comparative studies that no significant differences can be expected in the clinical outcome and in terms of the re-rupture rate. When there is early passive exercise, the full range of motion (ROM) is also achieved more quickly, particularly in terms of flexion. In a detailed evaluation of the meta analyses and our own additional review of the literature, a total of four Level I studies were identified that support the recommendation of early passive mobilization [2, 8, 20, 22].
By contrast, early aggressive active exercise should be avoided since this negatively impacts the healing process [20]. In order to protect patients from excessive strain outside the therapy setting, an aid can be used to immobilize the arm. Based on the timeframe of tendon healing mentioned above, the length of immobilization varies widely between 4 and 8 weeks [2, 4, 14, 21, 22, 29]. There are no prospective studies that deal only with the length of immobilization.
While the duration of immobilization is the subject of debate, immobilization in slight abduction is predominantly preferred by the experts surveyed as this increases blood circulation in the tendon and reduces the strain on the reconstruction [38]. Gerber et al. [15] and Thomopoulos et al. [45] were also able to show in animal models that a position that lowers the strain on the tendon reconstruction has a positive effect on the orientation of the collagen fibers and the elasticity of the tendon. Orthoses are, in principle, suitable for lowering the activity of the RC muscles. This was proven by Alenabi et al. [1] in an electromyographic study. When the elbow and hand were moved in a splint, the activity of the RC muscle was measured at no more than 10% of normal activity. There are no clinical investigations that specifically look at the type of orthoses used. The German catalog of medical aids allows both the use of arm slings and abduction pillows with a varying abduction of 15–45° for post-treatment after an RCR.
Conclusions
Early passive, postoperative exercise can be used without indicating an increased rate of disruption of the healing process or ruptures. Employing an orthosis can protect against active strain that is applied too early. There are no evidence-based recommendations regarding the length of time that postoperative immobilization should last. The use of an arm abduction pillow can be considered (see Table 5 for DVSE expert opinions on immobilization).
Physical therapy
Cryotherapy, electrotherapy and exercise in an exercise pool are frequent methods of physical therapy that are used following an RCR. In a randomized clinical trial (RCT) with 50 patients conducted in 1996, Speer et al. [43] investigated the effects of using cryotherapy systems after a variety of shoulder operations, including RCR. Continuous cryotherapy leads to a reduction in pain, a reduced need for pain killers and better sleep quality in the night after the operation. When cryotherapy was used (4 to 6 times per day depending on patient requirements) there was less pain when the arm was at rest and in motion in the 10 days following the operation. In another RCT, the same working group also observed clinically relevant effects on pain in the cryotherapy group when at rest and when physical strain was placed on the shoulder following open and arthroscopic shoulder operations (n = 70; water temperature 7–13 °C; length it was worn: continuously 48 h postoperatively; at night on days 3–7; daily 2–4 h on days 8–21 followed by exercise therapy; [42]). Speer’s group also demonstrated that continuous cryotherapy directly following reconstruction of the RC reduced the temperature in the glenohumeral joint and subacromial space by around 0.5–1.0 °C [35].
Blum et al. [4] compared two types of electrotherapy in an RCT with 22 patients who received RC reconstruction. The control group received 2 × 1 h of electrotherapy per day in connection with physiotherapy that started 6–8 weeks post-op. The intervention group received the same length of sham electrotherapy and physiotherapy that began 8 weeks after the operation.
In contrast to the control group, movement improved in the intervention group by around 10° 45 and 90 days after the operation, however strength did not. The methodological quality of the study should be regarded critically as the authors had a relevant conflict of interest.
A non-randomized study indicates that additional group sessions of aquatic theraphy (starting 10 days after reconstruction) have a positive effect on passive movement (anteversion and external rotation), pain and activities of daily living (Western Ontario Rotator Cuff Score) 3 and 6 weeks, though not 12 weeks, post operation [5]. However, the effects were slight and could also be the result of an overall higher amount of active intervention in the aquatic therapy group.
Conclusions
Cryotherapy is recommended in the first 3 weeks following RCR in order to support rehabilitation and, in particular, to treat pain [43]. Based on current published studies, no clear recommendation can be made for or against electrotherapy, aquatic therapy, the application of heat, massages, therapeutic ultrasound, extracorporeal shockwave therapy and injections of hyaluronic acid [4, 5, 21, 34].
Individual studies indicate a potential benefit of electrotherapy and group training in an exercise pool (see Table 6 for DVSE expert opinions on physical therapy).
Continuous passive motion
Continuous passive motion therapy with a motorized CPM machine is one of the most frequently used elements of treatment following an operation on a shoulder joint, and particularly after RCR. The passive motion machine typically serves to mobilize the joint shortly after the operation without the patient having to actively support the extension of motion.
Currently scientific literature only contains two reviews [3, 10] and one prospective randomized study [14]. The review by Baumgarten et al. [3] is based on two studies in which a CPM machine was used on 26 patients [37] and 31 patients respectively [28]. Both investigations compared physical therapy treatment (manual passive exercise) to the use of a CPM machine. Baumgarten et al. [3] concluded that the validity of the data is limited due to its poor methodological quality and provides insufficient evidence for the development of an evidence-based rehabilitation protocol. CPM treatment was not found to be superior. Du Plessis et al. [10] compared the effects of standard physical therapy to CPM therapy combined with physical therapy. The group of patients receiving CPM combined with physical therapy treatment, were given passive, isometric and actively supported exercises, shoulder mobilization and strength training. Manual passive mobilization, active exercise, and therapist coordinated self-exercise were used in the group that received standard physical training. Data on the range of movement, muscle strength, and pain reduction was collected, and studies previously conducted by Raab et al. [37] and Lastayo et al. [28], as well as a paper by Michael et al. [33] were also included. The authors of the review concluded that the use of a CPM machine in combination with physical therapy as part of follow-up treatment after an RCR can be regarded as safe [10]. A paper by Garofalo et al. [14] looked at 100 patients, comparing a standard program of passive exercise (therapist coordinated self-exercise: three series with 10 repetitions, pendulum movement, passive abduction, flexion and external rotation) to the same program with the addition of a CPM chair. This was applied for two hours a day for 4 × 30 min. In this comparison, the additional use of a CPM chair led to an improvement in results. It remains unclear, however, whether the device itself or the additional movement produced this effect [14].
Conclusions
Based on these studies, no recommendation can be made with a high level of evidence for or against the use of CMP therapy following RCR, and not for the length of time, frequency and intensity of the CPM treatment. It should be noted, however, that passive motion exercise does not negatively impact the healing process (see Table 7 for the DVSE expert opinions on CPM).
Self-exercise
In addition to CPM, self-exercise is another important component of post-operative follow-up treatment which is used to varying degrees [8, 11, 18, 29]. There are major differences in point in time, intensity, type of exercise and supporting measures. Patients can be instructed through written directions, videos and/or receive instruction from the physiotherapist (PT). Roddey et al. [39] found there was no significant difference in the post-operative outcome when the instructions were given by the PT or by video.
Pendulum exercises were often described in the first post-operative phase. Biomechanical studies have shown that it is important to carry them out correctly so that there is low electromyographic activity (EMG) in the reconstructed RC (see below, [32]). The lowest activity was recorded in small pendulum circles (d = 20 cm) with an initiation of the arm movement by moving the torso and not by using the shoulder muscles themselves [32]. Additional use of a 1.5 kg weight on the hanging arm increases the EMG activity of M. supraspinatus and M. infraspinatus, though not to a statistically significant degree [12]. Furthermore, mobilization exercises (with the help of the contra-lateral arm) and, in later phases, muscle activation/strengthening exercises using simple devices (e. g. theraband, dumbbells) are other primary forms of self-exercise [8, 11, 46]. There is no homogeneous data on the extent of the passive mobilization and when to start it [2, 8, 21, 29]. The question of whether self-exercise, in addition to physiotherapy, has a positive effect cannot be sufficiently answered. Both are combined in many published studies, however a direct comparative study currently does not exist [1, 11, 31, 33, 46]. Scientific literature contains only two randomized controlled trials that look at self-exercise versus physiotherapy exercise [3]. In their Level II study, Hayes et al. [17] were able to randomize 58 patients into two control groups. After both groups received instructions on the self-exercise program in the first week, one group subsequently received physiotherapy treatment while the other group continued to do the self-exercise program. No significant differences in ROM, strength measurement and shoulder scores were found at any of the follow-up treatments (6, 12 and 24 weeks). Critical aspects of the study include the low number of cases, the high conversion rate from the self-exercise group to the physiotherapy group (n = 9) and the high drop-out rate (27%).
The second study by Lee et al. conducted a clinical and neurophysiological examination of 11 patients per group at days 20 and 40 post operation [29]. The results showed significantly better active mobility at both follow-ups in the group receiving physiotherapy. This group also achieved a significant improvement in the activation of motor units in the EMG; this was not detected in the self-exercise group. In this study the low number of cases, the short follow-up period and an absence of clinical scores should be viewed critically.
Conclusions
No Level I-based recommendation for or against the use of self-exercise can be made, however, based on the available studies, its use can be considered (see Table 7 for DVSE expert opinions on self-exercise).
Physiotherapy and the phase model
Rehabilitation phases/protocols (time- and criteria-based)
In order to enable continuous progression of rehab-treatment, the post-operative process should be divided into different phases. The available literature usually breaks the process down into four phases. Thus, the different treatment focuses and the corresponding targets can be usefully classified [23, 27, 40, 44].
-
The first phase is the time directly after the operation until week 6. During this time mainly passive and assistive exercises are conducted.
-
This is followed by Phase 2 that lasts a further 6 weeks during which active functions are regained (week 7–12 post operation).
-
Phase 3, strength building, starts in the third post-operative month (month 3 and 4).
-
This is concluded by Phase 4 which includes the return to sports (Table 8; [20]).
The timeline is aligned with the general phases of wound healing and the time it takes for tissue to heal, as identified in animal studies. These time markers define the framework for the follow-up treatment phases. There is a consensus that rehabilitation should be improved both in terms of time and criteria [9]. The literature defines no precise criteria that should act as the specific criteria which the patient should fulfil before moving on to the next rehabilitation phase. However, the “International Classification of Functioning, Disability and Health” (ICF) is a good basis for identifying targets. Orientational criteria are assigned to each phase as listed in Table 8.
The four-phase model is structured as follows: Phase 1 lasts 6 weeks starting on the first day after the operation [20]. During this time, the key targets include achieving good tendon healing without post-operative adhesions and, above all, reducing pain for the patient. Throughout Phase 1 the shoulder should be immobilized in a position at15–45° abduction using an orthosis/sling/brace which is only taken off during physiotherapy and for hygiene purposes [8]. Only a passive exercise program is allowed until the end of the fourth week after the operation. Then, depending on the amount of pain the patient is still in, assistive exercise can be integrated into the treatment program [5, 33, 46]. Nevertheless, the extent of motion is limited to 30° external rotation, 90° flexion and abduction in a pain-free range [29, 37]. Adduction should be avoided both in a passive and assistive fashion. The core exercises in this phase are pendulum and scapula-thoracic exercises. All of the exercises to passively expand elevation are only allowed in a closed chain [1]. Only the exercising of the adjacent joints, elbow, hand and fingers is active and allowed in an open chain [1, 26, 46]. After 6 weeks and at the end of Phase 1, a passive flexion up to 90°, a passive internal and external rotation with adjacent scapula up to 45° and a passive abduction, also with an adjacent scapula up to 90° on the operated side, should be possible. The movement should be symmetrical to the opposite side and pain free.
The activities of Phase 2 are done until week 12 following the operation. The goals of this phase include tissue healing, achieving a full passive range of movement and the development of dynamic shoulder stabilization. In this phase of tendon healing and remodeling, only “low level loading” is allowed. At the same time, scar mobilization is an important element to prevent adhesions. By the end of this phase, the full range of movement can be trained in an active-assistive fashion and an active increase in movement against the force of gravity can start. This targets the improvement of the kinematics of the shoulder joint [9].
Twelve weeks after the operation the patient should also actively achieve the degree of motion that was achieved passively up to this point in time. It should be noted that by now there should no longer be any scapulothoracic dysfunction [9]. Once there is sufficient glenohumeral and scapulothoracic movement, the therapy can move on to Phase 3 [9].
In Phase 3 (month 3 and 4 post operation) the full active range of movement and dynamic shoulder stabilization should be achieved. At this point in time, tendon healing should have progressed enough to integrate strengthening and stretching as additional elements in this phase so that patients can regain functional activity and participate in their professional and social lives [9]. Light functional exercises and mobilization/strengthening exercises using a pulley with low weights are a good way to do this at this time [26, 46]. Push-ups against the wall [5, 11] and bicep and tricep exercises with low free weights or a resistance band are once again permitted [46].
At the end of post-operative month 4 the patient should have regained full functional movement within the pain-free range and be able to perform activities of daily living (ADL) without pain [9]. At this point in time, around 75% of normal strength and endurance has been reestablished [9]. When there is sufficient strength in the RC to carry out ADL cleanly and without pain, Phase 4 can start. In the fourth and final phase, which extends up to 6 months after the operation, training focuses on maintaining a final and pain-free active range of movement, improving strength and flexibility, and improving endurance and explosive power [9]. Regaining functional activities and reestablishing kinematics related to sports, daily life and work are the aims of this final phase as defined by the ICF [9]. A return to sports isn’t permitted until the end of this final phase and until mobility and strength are symmetrical with the opposite side. Further requirements are normal scapulothoracic mobility and no pain at rest and during activity ([46]; see Table 7 and 9 for DVSE expert opinions on physiotherapy and the phase model).
Conclusions for clinical practice
Today, RCR is an established standard procedure. The post-operative follow-up treatment period is expected to be long and time-consuming. The contents and concepts of therapy are, thus, applied in different ways and controversially discussed. The number of publications on the subject is therefore high. Unfortunately, not all of the papers fulfil the required quality criteria of evidence-based medicine.
Since 2004 one guideline, four reviews and 17 original papers have been identified that serve as the basis for establishing structured follow-up treatment. For some treatments, clear recommendations can be derived. These include early passive exercise, using cryotherapy to reduce the pain, self-exercise and the use of orthoses. Despite this, there are still questions that cannot be answered conclusively based on the available literature. When all of the results were looked at together, a basic concept that was solid and valid could nevertheless be created which was summarized in a four-phase model. The main points of this model were supported and supplemented for the first time through collected and pooled expert opinions from the DVSE expert society.
References
Alenabi T, Jackson M, Tetreault P et al (2013) Electromyographic activity in the immobilized shoulder musculature during ipsilateral elbow, wrist, and finger movements while wearing a shoulder orthosis. J Shoulder Elbow Surg 22:1400–1407
Arndt J, Clavert P, Mielcarek P et al (2012) Immediate passive motion versus immobilization after endoscopic supraspinatus tendon repair: a prospective randomized study. Orthop Traumatol Surg Res 98:S131–S138
Baumgarten KM, Vidal AF, Wright RW (2009) Rotator cuff repair rehabilitation: a level I and II systematic review. Sports Health 1:125–130
Blum K, Chen AL, Chen TJ et al (2009) Repetitive H‑wave device stimulation and program induces significant increases in the range of motion of post operative rotator cuff reconstruction in a double-blinded randomized placebo controlled human study. BMC Musculoskelet Disord 10:132
Brady B, Redfern J, Macdougal G et al (2008) The addition of aquatic therapy to rehabilitation following surgical rotator cuff repair: a feasibility study. Physiother Res Int 13:153–161
Chan K, Macdermid JC, Hoppe DJ et al (2014) Delayed versus early motion after arthroscopic rotator cuff repair: a meta-analysis. J Shoulder Elbow Surg 23:1631–1639
Conti M, Garofalo R, Delle Rose G et al (2009) Post-operative rehabilitation after surgical repair of the rotator cuff. Chir Organi Mov 93(Suppl 1):S55–S63
Cuff DJ, Pupello DR (2012) Prospective randomized study of arthroscopic rotator cuff repair using an early versus delayed postoperative physical therapy protocol. J Shoulder Elbow Surg 21:1450–1455
Dreinhofer KE, Schuler S, Schafer M et al (2014) Rehabilitation concepts and return to sport after interventions on the shoulder. Orthopäde 43:256–264
Du Plessis M, Eksteen E, Jenneker A et al (2011) The effectiveness of continuous passive motion on range of motion, pain and muscle strength following rotator cuff repair: a systematic review. Clin Rehabil 25:291–302
Duzgun I, Baltaci G, Atay OA (2011) Comparison of slow and accelerated rehabilitation protocol after arthroscopic rotator cuff repair: pain and functional activity. Acta Orthop Traumatol Turc 45:23–33
Ellsworth AA, Mullaney M, Tyler TF et al (2006) Electromyography of selected shoulder musculature during un-weighted and weighted pendulum exercises. N Am J Sports Phys Ther 1:73–79
Galatz LM, Charlton N, Das R et al (2009) Complete removal of load is detrimental to rotator cuff healing. J Shoulder Elbow Surg 18:669–675
Garofalo R, Conti M, Notarnicola A et al (2010) Effects of one-month continuous passive motion after arthroscopic rotator cuff repair: results at 1‑year follow-up of a prospective randomized study. Musculoskelet Surg 94(Suppl 1):S79–S83
Gerber C, Schneeberger AG, Perren SM et al (1999) Experimental rotator cuff repair. A preliminary study. J Bone Joint Surg Am 81:1281–1290
Gimbel JA, Van Kleunen JP, Williams GR et al (2007) Long durations of immobilization in the rat result in enhanced mechanical properties of the healing supraspinatus tendon insertion site. J Biomech Eng 129:400–404
Hayes K, Ginn KA, Walton JR et al (2004) A randomised clinical trial evaluating the efficacy of physiotherapy after rotator cuff repair. Aust J Physiother 50:77–83
Holmgren T, Oberg B, Sjoberg I et al (2012) Supervised strengthening exercises versus home-based movement exercises after arthroscopic acromioplasty: a randomized clinical trial. J Rehabil Med 44:12–18
Hultenheim Klintberg I, Gunnarsson AC, Styf J et al (2008) Early activation or a more protective regime after arthroscopic subacromial decompression—a description of clinical changes with two different physiotherapy treatment protocols—a prospective, randomized pilot study with a two-year follow-up. Clin Rehabil 22:951–965
Keener JD, Galatz LM, Stobbs-Cucchi G et al (2014) Rehabilitation following arthroscopic rotator cuff repair: a prospective randomized trial of immobilization compared with early motion. J Bone Joint Surg Am 96:11–19
Kim JY, Lee JS, Park CW (2012) Extracorporeal shock wave therapy is not useful after arthroscopic rotator cuff repair. Knee Surg Sports Traumatol Arthrosc 20:2567–2572
Kim YS, Chung SW, Kim JY et al (2012) Is early passive motion exercise necessary after arthroscopic rotator cuff repair? Am J Sports Med 40:815–821
Korsukéwitz C (2008) Evidenz und Qualität in der Rehabilitation: Die Leitlinien der Deutschen Rentenversicherung. In: Bund DR (ed) 17. Rehabilitations-wissenschaftliches Kolloquium. Bremen, pp 31–32
Korsukewitz C, Rose S, Schliehe F (2003) The significance of clinical guidelines for rehabilitation. Rehabilitation (Stuttg) 42:67–73
Korsukéwitz CRS, Schliehe F (2003) Zur Bedeutung von Leitlinien für die Rehabilitation. Rehabilitation (Stuttg) 42:67–73
Krischak G, Gebhard F, Reichel H et al (2013) A prospective randomized controlled trial comparing occupational therapy with home-based exercises in conservative treatment of rotator cuff tears. J Shoulder Elbow Surg 22:1173–1179
Lapner PL, Sabri E, Rakhra K et al (2012) A multicenter randomized controlled trial comparing single-row with double-row fixation in arthroscopic rotator cuff repair. J Bone Joint Surg Am 94:1249–1257
Lastayo PC, Wright T, Jaffe R et al (1998) Continuous passive motion after repair of the rotator cuff. A prospective outcome study. J Bone Joint Surg Am 80:1002–1011
Lee BG, Cho NS, Rhee YG (2012) Effect of two rehabilitation protocols on range of motion and healing rates after arthroscopic rotator cuff repair: aggressive versus limited early passive exercises. Arthroscopy 28:34–42
Li S, Min SX, Zhang H et al (2010) Effect of continuous passive motion on basic fibroblast growth factor expression during tendon-bone repair after surgical repair of acute rupture of the supraspinatus tendon in rabbits. J South Med Univ 30:1020–1023
Lisinski P, Huber J, Wilkosz P et al (2012) Supervised versus uncontrolled rehabilitation of patients after rotator cuff repair-clinical and neurophysiological comparative study. Int J Artif Organs 35:45–54
Long JL, Ruberte Thiele RA, Skendzel JG et al (2010) Activation of the shoulder musculature during pendulum exercises and light activities. J Orthop Sports Phys Ther 40:230–237
Michael JW, Konig DP, Imhoff AB et al (2005) Efficiency of a postoperative treatment after rotator cuff repair with a continuous passive motion device (CPM). Z Orthop Ihre Grenzgeb 143:438–445
Oh CH, Oh JH, Kim SH et al (2011) Effectiveness of subacromial anti-adhesive agent injection after arthroscopic rotator cuff repair: prospective randomized comparison study. Clin Orthop Surg 3:55–61
Osbahr DC, Cawley PW, Speer KP (2002) The effect of continuous cryotherapy on glenohumeral joint and subacromial space temperatures in the postoperative shoulder. Arthroscopy 18:748–754
Peltz CD, Dourte LM, Kuntz AF et al (2009) The effect of postoperative passive motion on rotator cuff healing in a rat model. J Bone Joint Surg Am 91:2421–2429
Raab MG, Rzeszutko D, O’connor W et al (1996) Early results of continuous passive motion after rotator cuff repair: a prospective, randomized, blinded, controlled study. Am J Orthop (Belle Mead, NJ) 25:214–220
Rathbun JB, Macnab I (1970) The microvascular pattern of the rotator cuff. J Bone Joint Surg Br 52:540–553
Roddey TS, Olson SL, Gartsman GM et al (2002) A randomized controlled trial comparing 2 instructional approaches to home exercise instruction following arthroscopic full-thickness rotator cuff repair surgery. J Orthop Sports Phys Ther 32:548–559
Ross D, Maerz T, Lynch J et al (2014) Rehabilitation following arthroscopic rotator cuff repair: a review of current literature. J Am Acad Orthop Surg 22:1–9
Shen C, Tang ZH, Hu JZ et al (2014) Does immobilization after arthroscopic rotator cuff repair increase tendon healing? A systematic review and meta-analysis. Arch Orthop Trauma Surg 134:1279–1285
Singh H, Osbahr DC, Holovacs TF et al (2001) The efficacy of continuous cryotherapy on the postoperative shoulder: a prospective, randomized investigation. J Shoulder Elbow Surg 10:522–525
Speer KP, Warren RF, Horowitz L (1996) The efficacy of cryotherapy in the postoperative shoulder. J Shoulder Elbow Surg 5:62–68
Tashjian RZ (2012) Epidemiology, natural history, and indications for treatment of rotator cuff tears. Clin Sports Med 31:589–604
Thomopoulos S, Williams GR, Soslowsky LJ (2003) Tendon to bone healing: differences in biomechanical, structural, and compositional properties due to a range of activity levels. J Biomech Eng 125:106–113
Van Der Meijden OA, Westgard P, Chandler Z et al (2012) Rehabilitation after arthroscopic rotator cuff repair: current concepts review and evidence-based guidelines. Int J Sports Phys Ther 7:197–218
Zhang S, Li H, Tao H et al (2013) Delayed early passive motion is harmless to shoulder rotator cuff healing in a rabbit model. Am J Sports Med 41:1885–1892
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
C. Jung, L. Tepohl, R. Tholen, K. Beitzel, S. Buchmann, T. Gottfried, C. Grim, B. Mauch, G. Krischak, H. Ortmann, C. Schoch and F. Mauch declare that they have no competing interests.
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
This is an English translation of the publication Rehabilitation nach Rotatorenmanschettenrekonstruktion. Eine Arbeit der Kommission Rehabilitation der Deutschen Vereinigung für Schulter und Ellenbogenchirurgie e. V. (DVSE) in Zusammenarbeit mit dem Deutschen Verband für Physiotherapie (ZVK) e. V., dem Verband Physikalische Therapie, Vereinigung für die physiotherapeutischen Berufe (VPT) e. V. und der Sektion Rehabilitation—Physikalische Therapie der Deutschen Gesellschaft für Orthopädie und Unfallchirurgie e. V. (DGOU) Obere Extremität 2016, 11:16–31 DOI https://doi.org/10.1007/s11678-015-0346-9.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Jung, C., Tepohl, L., Tholen, R. et al. Rehabilitation following rotator cuff repair. Obere Extremität 13, 45–61 (2018). https://doi.org/10.1007/s11678-018-0448-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11678-018-0448-2