Abstract
Acorn production in oak (Quercus spp.) shows considerable inter-annual variation, known as masting, which provides a natural defence against seed predators but a highly-variable supply of acorns for uses such as in commercial tree planting each year. Anthropogenic emissions of greenhouse gases have been very widely reported to influence plant growth and seed or fruit size and quantity via the ‘fertilisation effect’ that leads to enhanced photosynthesis. To examine if acorn production in mature woodland communities will be affected by further increase in CO2, the contents of litter traps from a Free Air Carbon Enrichment (FACE) experiment in deciduous woodland in central England were analysed for numbers of flowers and acorns of pedunculate oak (Quercus robur L.) at different stages of development and their predation levels under ambient and elevated CO2 concentrations. Inter-annual variation in acorn numbers was considerable and cyclical between 2015 and 2021, with the greatest numbers of mature acorns in 2015, 2017 and 2020 but almost none in 2018. The numbers of flowers, enlarged cups, immature acorns, empty acorn cups, and galls in the litter traps also varied amongst years; comparatively high numbers of enlarged cups were recorded in 2018, suggesting Q. robur at this site is a fruit maturation masting species (i.e., the extent of abortion of pollinated flowers during acorn development affects mature acorn numbers greatly). Raising the atmospheric CO2 concentration by 150 μL L−1, from early 2017, increased the numbers of immature acorns, and all acorn evidence (empty cups + immature acorns + mature acorns) detected in the litter traps compared to ambient controls by 2021, but did not consistently affect the numbers of flowers, enlarged cups, empty cups, or mature acorns. The number of flowers in the elevated CO2 plots’ litter traps was greater in 2018 than 2017, one year after CO2 enrichment began, whereas numbers declined in ambient plots. Enrichment with CO2 also increased the number of oak knopper galls (Andricus quercuscalicis Burgsdorf). We conclude that elevated CO2 increased the occurrence of acorns developing from flowers, but the putative benefit to mature acorn numbers may have been hidden by excessive pre- and/or post-dispersal predation. There was no evidence that elevated CO2 altered masting behaviour.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cumulative net anthropogenic carbon dioxide (CO2) emissions have amounted to 2400 ± 240 Gt since 1850, 42% of this between 1990 and 2019, raising atmospheric CO2 concentrations to a global annual average of 410 parts per million in 2019 (IPCC 2023). These emissions together with those of other greenhouse gases increased global surface temperatures by 1.1 °C above 1850 − 1900 values in the period 2011 − 2020, with anthropogenic climate change affecting every region of our planet (IPCC 2023). Anthropogenic-induced climate change has well-reported effects and will also have future impacts on forest survival and composition (Flannigan et al. 2000; Ibáñez et al. 2006; Sturrock et al. 2011; Khaine and Woo 2015). It is also established that increased CO2 will have a direct fertilization effect (Zhu et al. 2016; Ruehr et al. 2023). Under elevated CO2, plants show increased photosynthetic rates and decreased CO2 loss via photorespiration, along with a reduced stomatal conductance that results in increased water use efficiency (Drake et al. 1997; Long et al. 2004; Gardner et al. 2022a). Plants may acclimate to greater CO2 to some extent over time, the degree of acclimation varying with temperature and leaf nitrogen content, but nonetheless a benefit to assimilate production is retained (Wheeler et al. 2004). This CO2 fertilisation effect leads to increased plant growth (Ainsworth and Long 2005, 2021; De Graaff et al. 2006), induces alterations in plant structure (Pritchard et al. 1999), and affects reproductive outcomes by increasing flowers and seed production, although the strength of these responses vary amongst species (Jablonski et al. 2002). The literature on the effects of differences in CO2 concentration on plants tends to rely greatly on small-scale studies of individual plants or closely-confined experiments (Wand et al. 1999; Poorter and Navas 2003). This has begun to change since the advent of Free Air Carbon Enrichment (FACE) experiments (Hendrey et al. 1999; De Graaff et al. 2006; DOE 2020), in which CO2 levels are elevated via a method of open-air CO2 enrichment (Ainsworth and Long 2021) within the field environment. Whilst FACE experiments have mostly agreed with prior findings of elevated CO2 under experimental conditions in many species (Kimball et al. 2002; Long et al. 2004), the advantage of FACE is that it subjects intact ecosystem patches to elevated CO2, so that it is testing a community response rather than the response of (often potted) plants. Hence, FACE experiments are important extensions to work on potted plants not just because of the size of trees but also because all the biotic and abiotic drivers are in play simultaneously. However, such studies with elevated CO2 are difficult to perform on mature trees due to their large size, and so there is a need for further research on the topic.
Fruit and seed production by plants is a resource-intensive process that uses large amounts of carbohydrates derived from photosynthesis, often competing with vegetative growth (Obeso 2002). Many long-lived perennial plants employ a reproductive strategy known as "masting” defined, in part, by large inter-annual variation in flowers or seeds/fruits produced (Kelly 1994). One of several mechanistic controls of masting in oak (Quercus spp.) has previously been attributed to variations in the rates of fruit maturation or abortion rather than the quantity of flowers produced (Pearse et al. 2016; Hacket-Pain 2021). Recent research, however, suggests that masting species may practice different adaptive strategies of either “fruit maturation” or “flower masting”, with the prevalence of one strategy over the other dependent on the environmental conditions of the site (Fleurot et al. 2023). Masting is hypothesized to have evolved as a predator satiation strategy and may be mechanistically controlled by resource dynamics (Isagi et al. 1997; Koenig and Knops 2005). The significance of fruit production in mature communities for tree demography and the maintenance of food webs makes it important to determine the effects of elevated CO2 on seed production in masting species empirically. An increase in baseline seed production could ultimately result in lower plant fitness if seed supply is surplus to requirement for successful propagation, preventing the herbivore limiting effects of masting’s seed predator satiation—starving cycle (Bogdziewicz et al. 2020). On the other hand, a decline in seed production may damage natural regeneration or reduce seed supply for human-managed regeneration projects (Bole 2022).
Isotope studies with several masting tree species have concluded that 100% of the carbon resources for fruiting are provided by fresh photosynthate—rather than from stored carbon resources—and so elevated CO2 may influence tree fruiting promptly and directly (Hoch et al. 2013). Experimental evidence supports this suggestion. One of the few studies reported on this topic was performed on a plantation of 13-year-old Loblolly pine (Pinus taeda L.) at the Duke Forest FACE site, North Carolina, USA, in which the numbers of cones increased up to three fold in individual trees under elevated CO2 (+ 200 μL L−1) compared to ambient CO2 (LaDeau and Clark 2001; Way et al. 2010). Similarly, in the Aspen FACE site in Rhinelander, Wisconsin, USA, flower numbers in 10-year-old paper birch (Betula papyrifera Marshall) trees grown from seedlings under elevated CO2 (+ 200 μL L−1) were increased by up to 100% and 260% in each of two years compared to the control group, while seed mass was only increased in the first year of the experiment (Darbah et al. 2008). In agreement with the above, modelling studies have linked elevated CO2 to a stronger influence on multi-decadal increases in flowering rates in tropical species (Pau et al. 2018). Similarly, an in situ growth chamber study conducted on a mature scrub oak community (Quercus myrtifolia Willd., Q. chapmanii Sarg., and Q. geminata Small) saw increases in acorn production with elevated CO2 (+ 325 μL L−1) but only for the dominant species in the environment (Stiling et al. 2004). On the other hand, it has also been hypothesised that trees may acclimatise to elevated CO2 over time, presenting down-regulation of photosynthesis (Ainsworth and Long 2005); mineral nutrients might also become limiting over the longer term (Hoch et al. 2013; Palacio et al. 2014). To date, the few FACE studies examining the effect of elevated CO2 upon the reproductive behaviour of masting trees have been performed on comparatively young trees; < 19 years old (LaDeau and Clark 2001; Darbah et al. 2008; Way et al. 2010), but not mature communities. Exposing an established tree community to a step-change in atmospheric CO2 is unlikely to have the same effect on propagule production as in trees grown under elevated CO2 as juveniles. Whether the masting behaviour of mature trees—typically slower-growing and with greater internal nutrient reserves—will respond differently to elevated CO2 or not remains unknown.
To address this question we investigated the influence of increased atmospheric CO2 concentration on flower and acorn production of the masting species (Askeyev et al. 2005; Wesołowski et al. 2015) pedunculate oak (Quercus robur L.). Reproductive material was recorded from litter traps over seven years within the Birmingham Institute of Forest Research (BIFoR) Free-Air Carbon Enrichment (FACE) facility, in which mature trees were exposed to elevated CO2. We tested the null hypotheses that counts of each of the number of flowers, enlarged cups, immature acorns, mature acorns, empty cups, and galls within the litter traps were unaffected by year, by CO2 treatment, or by the interaction of these factors.
Materials and methods
Study site
The Birmingham Institute of Forest Research (BIFoR) has maintained a Free-Air Carbon Enrichment (FACE) facility at Mill Haft, Staffordshire, UK (52°48′3.6″ N, 2°18′0″ W) since 2015, with CO2 treatments beginning in April 2017. Mill Haft is a 19.1 ha deciduous woodland in a temperate maritime climate; the woodland is dominated by Q. robur L., planted around 1850, in the upper canopy and Corylus avellana L. in the understorey (Hart et al. 2019; MacKenzie et al. 2021) alongside self-seeded Acer pseudoplantanus L. and Crataegus monogyna Jacq. of varying ages.
The site contains three experimental treatments divided equally across nine experimental areas, each approximately 30 m in diameter. These comprise of three ‘elevated CO2’ arrays maintained at + 150 μL L−1 above ambient CO2 (eCO2), three control arrays at ambient CO2 (aCO2) which blow ambient air collected and redistributed from the site, and three undisturbed woodland areas (i.e., no CO2 array infrastructure, uCO2). The FACE arrays have operated during daylight hours throughout the growing season from initial budburst to leaf fall (early April to late October) and the CO2 treatments since early April 2017 to the present.
There is significant spatial variability in soil volumetric water content at the site, with the undisturbed patches (uCO2) considerably wetter than the other two treatment groups (MacKenzie et al. 2021). Soil pH and phosphate contents for the treatment arrays aCO2 and eCO2 (only), recorded once in 2021, were broadly similar with the aCO2 array having slightly more phosphate but not significantly so (aCO2: phosphate = 2.63 ± 0.96 μg PO4 P/g, pH = 4.41 ± 0.05. eCO2: phosphate = 1.36 ± 0.48 μg PO4 P/g, pH = 4.26 ± 0.5). For further details of the site and the long-term experiment see Hart et al. (2019).
Data collection
Three litter traps each 1 m2 were placed within each of the nine arrays from 2015. This was changed to six litter traps of 0.25 m2 per array from 2020 with no detectable impact on amounts per unit area (see below). The litter traps were in place all year round and their contents were collected at least once a month during acorn fall between August and October each year. The Q. robur reproductive material collected from litter traps from 2015 to 2021 was separated, classified, counted, and totalled within each year. The reproductive material was classified into six categories encompassing acorn development and predation: female flowers (unpollinated or aborted flowers with no visible acorn development); enlarged cups (swollen cups and visible premature acorns); immature acorns (immature acorns with length < 14 mm and diameter < 7 mm); mature acorns (fully mature acorns); empty cups (large empty acorn cups with acorns missing); and galls (acorn development prevented by insect attack). A seventh category was calculated for all evidence of acorns. This combined observations for immature and mature acorns with an estimate of seed predation (i.e., empty cups) and provided a best estimate of total acorn numbers because empty cups are often all that remains after post-dispersal seed predation (Martínez-Baroja et al. 2019). To allow comparison between years in which trap number and size differed all data was standardised to the amount of reproductive material per 1 m2 of litter trap area. To do this, counts from litter traps of 0.25 m2 were multiplied by four (comparable to traps of 1 m2), and these and those of 1 m2 were averaged by the number of litter traps in each array (n = 3 or 6) to give the average count per 1 m2. The means for each collection date were then added to give the total amount of reproductive material produced by each array per 1 m2 across the whole year, with three arrays in each treatment (n = 3).
Data analyses
All analyses were performed using R statistical software (R Core Team 2021). Model assumptions were tested with the ggrplot and rootogram functions in the package ‘countreg’ and by examining diagnostic plots from the functions plotQQunif and plotResiduals in the package ‘DHARMa’. The best-fitting models were decided by comparing models’ Akaike Information Criterion (AIC) scores (Akaike 1998) and by comparing models via the vuong function and package. After the most appropriate family of model had been determined, the selection of explanatory variables to include were decided by AIC scores. The best fits were provided by Negative Binomial Distribution models in all cases, with the explanatory variables Treatment (i.e., array type) and Year, and these are presented here.
Negative Binomial Models were built with the glm.nb function in the package ‘MASS’. Initial models, with the factors Treatment and Year and the Treatment × Year interaction, were built separately for each of the seven categories of acorn development and predation as the response variables. The function Anova from the ‘car’ package was used as an omnibus test to see if there was a significant difference in reproductive material amongst treatments. If the default Anova (type 2) provided a significant interaction, then the model was run again as a type 3. The latter is preferred in the presence of a significant interaction (Langsrud 2003). Where significant differences were detected, post-hoc Tukey pairwise comparisons were conducted via the emmeans model function of the ‘emmeans’ package. To generate a baseline comparison, analyses of observations for the two years before the treatments began (2015 and 2016) were made. Further, as site conditions were found to be quite heterogeneous, especially of the ‘undisturbed’ plots with high soil volumetric water content, statistical analyses were also re-run with only the two treatments eCO2 and aCO2 (Supplementary Table 1).
Results
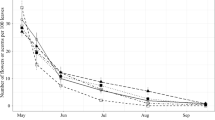
All categories of Q. robur reproductive material counted from the litter traps showed inter-annual variation, with high synchrony among the five reproductive material groupings (Fig. 1). Flower counts were consistently the most abundant category, whereas the other reproductive material varied in their rank order between groupings (Fig. 1). The lowest year for acorn production at Mill Haft was 2018 (Fig. 1). This was the case for all categories of acorn development (enlarged cups, immature acorns, mature acorns), and also for empty cups. Nevertheless, large numbers of flowers (aborted flowers with no visible acorn development) were collected in that low acorn production year, and post-hoc pairwise comparisons showed that flower numbers in 2018 were not significantly different from those during the mast years in 2015 (P = 0.99) nor 2020 (P = 0.54). These results demonstrate that masting behaviour was captured at the experimental site over the study period.
Variation in counts (logarithmic scale) of oak reproductive material from litter traps (mean of all three CO2 treatments) at the BIFoR FACE facility, Mill Haft, across seven years (CO2 treatments were provided from early April 2017 [vertical solid black line]): fully mature acorns (solid line, ■, FMA), immature acorns (long-dashed line, ●, ImA), empty cups (dotted line, ▲, EmC), enlarged cups (short-dashed line, Δ, EnC), flowers (dot-dash line, + , Flw), and all the reproductive material combined (two-dash line, □, All)
To test whether greater CO2 might either enhance, or mitigate the phenomenon of masting, the relative difference in mature acorn counts under eCO2 and aCO2 each year was regressed against the number in aCO2, viz. ( \(\frac{{eC0}_{2}- {aC0}_{2}}{{aC0}_{2}} \sim {aC0}_{2}\)); the argument being that if CO2 concentration affected masting then a trend would be detected. There was no trend in this relation across these five years (P = 0.87); that is the relative difference between the CO2 treatments did not vary with inter-annual variation in acorn production. Hence, masting was not affected by the differences in CO2 concentration. Similar analyses also showed no such relation for the relative effect of eCO2 on numbers of immature acorns (P = 0.74), the combined category of all evidence of acorns (P = 0.20), galls (P = 0.24), or of flowers (P = 0.29).
The counts of mature acorns, immature acorns, all evidence of acorns, and galls were affected significantly by the main effect of the CO2 treatments, whereas those of empty cups, enlarged cups and flowers were not (Table 1). The main effect of year (2017 − 2021) was significant for all seven variables, with a significant treatment × year interaction for immature acorns, empty cups, all evidence of acorns, and flowers: but not any other category. From this we conclude that CO2 treatment had a significant effect on Q. robur reproduction but for four of the seven categories of reproductive material this effect varied depending upon year.
To understand the effect of elevated CO2 on Q. robur reproductive behaviour and check if there was a baseline difference affecting the apparent treatment results found in Table 1, the mean counts of reproductive material were compared within and between each treatment for the periods before (2015 − 2016) and after the start of CO2 enrichment (2017 − 2021). For every category of reproductive material with a significant main effect of treatment and/or interaction of treatment × year except immature acorns (Table 1), the ‘undisturbed’ (uCO2) arrays provided the greatest number of counts throughout 2015 − 2021. Post hoc pairwise comparisons show the mean counts of all acorn evidence and galls to be significantly less (P < 0.05) in the aCO2 treatment compared with eCO2 and uCO2 (Fig. 2). This significantly greater quantity of all acorn evidence and galls (but no difference for mature acorns and empty cups) for elevated cf. aCO2 (Fig. 2) was confirmed by a re-analysis which omitted the undisturbed treatment (Supplementary Table 1). Furthermore, a significant increase (P < 0.05) in immature acorns and in all acorn evidence was detected between the periods before and after enrichment began specifically in the eCO2 treatment (Fig. 2). A significant increase (P < 0.05) in flower counts was also detected after the seasonal enrichment period but in both the ambient and elevated CO2 treatments (Fig. 2). A significant increase (P < 0.05) in the number of empty cups was also detected after enrichment began, specifically under the eCO2 treatment, but before the seasonal enrichment period began there were significantly fewer empty cups in the eCO2 plots than in the uCO2 and aCO2 treatments (Supplementary Table 2, Fig. 2). After enrichment began, however, the numbers of empty cups did not differ significantly amongst treatments (P > 0.05). Thus, these results suggest that enrichment with elevated CO2 resulted in an increase in the mean number of acorns developing compared to the pre-enrichment baseline period, whereas increases in the number of flowers could not be ascribed specifically to eCO2.
Variation in counts of Q. robur reproductive material from litter traps (mean of two years pre-treatment [2015 − 2016/light grey] and five years post-treatment [2017 − 2021/dark grey]) at the BIFoR FACE study at Mill Haft for the aCO2, eCO2 or uCO2 treatments. The vertical bars represent the mean ± standard error. Asterisks denote significant differences (P < 0.05) of a post hoc pairwise comparison between the periods before and after seasonal enrichment started (i.e. 2015 − 2016 cf. 2017 − 2021) within a treatment (aCO2, eCO2 or uCO2). Bars labelled with different letters (a, b) denotes significant differences (P < 0.05) between treatment groups during the two years before seasonal enrichment (2015 − 2016, light grey letters) and within the period after seasonal enrichment started (2017 − 2021, dark grey letters)
To investigate the significant interaction between CO2 treatment and year for several of the reproductive groups (Table 1), the significant differences of group means before and after enrichment began (Fig. 2), and how any effect of CO2 enrichment changed, the counts for each treatment per year were analysed. Analysis showed that before enrichment began (2015 and 2016), those woodland patches destined to be subjected to eCO2 had the lowest production of several reproductive material groups (Fig. 3), although the only significant pairwise comparison found was of empty cups in 2015 (aCO2 cf. eCO2, P < 0.001; eCO2 cf. undisturbed, P < 0.001). By the fifth year of CO2 enrichment (the last of the seven-year period studied, 2021), however, the elevated CO2 treatment provided the greatest numbers for immature acorns, empty cups, and all evidence of acorns (Fig. 3). Although this difference was not statistically significant between any treatment with post hoc pairwise comparison within 2021 (P = 0.64 to 0.99), a clear trend is nevertheless visible where counts in the elevated CO2 treatment transition from being the lowest of the three treatments (2017) to the highest (2021). Flower counts oscillated mostly in step between the treatment groups each year other than in 2018, one year after the CO2 treatments started. In that year, flower numbers in the elevated CO2 treatment were much greater than in 2017, whereas they decreased substantially between 2017 and 2018 in the ambient and undisturbed treatments (Fig. 3). Post hoc pairwise comparisons for flower numbers in 2018 showed a difference between eCO2 and undisturbed treatments (P < 0.05), but not between eCO2 and aCO2 (P = 0.86). These results suggest that the significant increase in developing acorns identified across the entire period of elevated CO2 enrichment (Fig. 2) is the result of a progressive effect over multiple years since enrichment began. In contrast, Q. robur flower production apparently demonstrated an immediate-but-transient response to elevated CO2 enrichment in 2017 − 2018.
Variation in counts of Q. robur reproductive material for mature acorns, immature acorns, empty cups, all evidence of acorns, enlarged cups, and flowers from litter traps at the BIFoR FACE study at Mill Haft across seven years for elevated CO2 (solid line), ambient CO2 (dashed line) and undisturbed plots (dotted line). The CO2 treatments were provided from early April 2017, shown by the vertical solid black line
Discussion
Masting
Masting is an important feature in the ecology of oak woodlands, which affects the ability of oak to regenerate naturally, and the supply of seed for tree nurseries. The counts of mature acorns and empty cups differed considerably amongst years (Table 1 and Fig. 1); mature acorns were most numerous in 2015, 2017, and 2020 but almost none were collected in 2018. The inter-annual variation at Mill Haft coincided with that for acorn production at other sites across the UK, in which 2015 and 2020 gave above-average numbers of acorns and 2018 very few. This is further evidence for the masting behaviour of high inter-annual variation in acorn output that is geographically synchronous, already well reported within oak species (Kelly 1994).
Oaks are said to be a “fruit maturation” species whereby it is the abortion of flowers and underdeveloped acorns that determines the level of acorn production each year rather than the number of flowers produced (Pearse et al. 2016; Bogdziewicz et al. 2019). This has been questioned recently; whether a population practices “fruit maturation” or “flower masting” may depend upon the climatic conditions experienced (Fleurot et al. 2023). All the reproductive material counted within the litter traps represents a flower that has either aborted or developed into an acorn. This total, along with the flower numbers, remained high each year, and consistently so compared to variation in the much lower counts of other reproductive material (Fig. 1). Thus, non-masting years were not caused by too few flowers; indeed, the failed acorn crop of 2018 followed an average number of flowers (Fig. 1) which was not significantly different from flower numbers during the mast years of 2015 (P = 0.99) and 2020 (P = 0.54). On average flowers were 53% of the count of reproductive material found each year, but 89% in 2018 highlighting the influence of early flower abortion during a failed year (Fig. 1). Hence, inter-annual variation in flowering (Table 1) may set an upper limit to acorn production in any one year but not markedly affect the numbers of acorns actually produced. We conclude that Q. robur masting is affected primarily by the success of reproductive processes that occur after flowering, consistent with the fruit maturation model. This mode of masting has been linked to the maritime climate of northern UK sites (see Fleurot et al. 2023).
The patterns of variation in the counts of reproductive material across the seven years were broadly consistent between underdeveloped acorns (enlarged cups or immature acorns) and evidence of developed acorns (mature acorns or empty cups) (Fig. 1). In 2018, when mature acorn production failed, however, enlarged cups (the very initial stage of acorn development) was the second most numerous category of reproductive material (Fig. 1). In this year the number of enlarged cups was statistically indistinguishable from those during the mast year of 2015 (2015 = 1.87 per m2 vs. 2018 = 0.56 per m2; P = 0.48). In contrast the counts were much reduced for mature acorns (2015 = 4.53 per m2 vs. 2018 = 0.03 per m2; P < 0.001) and empty cups (2015 = 4.42 per m2 vs. 2018 = 0.11 per m2; P < 0.001). Hence, it is likely that it is the extent of abortion of pollinated flowers early on in acorn development that determines the numbers of mature acorns produced. Fewer pollinated flowers developing acorns wastes less resources and it may be due to selective abortion of an unfavourable pollen source or may indicate limited pollen availability (Boavida et al. 2001). The early abortion of acorns in 2018 (relatively more enlarged cups to immature and mature acorns, Fig. 1) could also be the result of environmental factors, such as summer drought (Espelta et al. 2008) or herbivore-mediated resource limitation (Canelo et al. 2018). In this regard, 2018 and 2019 were years marked by intense defoliation and powdery mildew infection in Mill Haft (Mayoral et al. 2023), which may have also affected acorn production.
CO2 treatment effects
Among the treatments, the undisturbed plots produced the most reproductive material overall (Fig. 2). The two CO2 plots may have been limited in some way. This might have been due to lower soil nitrate or phosphate, important for early-stage flower development (Allen et al. 2017) and acorn development (Sever et al. 2023), or lower soil water (MacKenzie et al. 2021) which reduces mineral and nutrient uptake by trees (Skopp et al. 1990; Alam 1999); or more carbon might have been allocated to the roots and lower stems for increased growth or storage (Dickson and Tomlinson 1996). It is also possible that the two CO2 treatment areas may have been stressed from the increase in activity, such as trampling, during and after the construction of the enrichment infrastructure (Komatsu et al. 2007).
There was a significant interaction between CO2 treatment and year on flower numbers (Table 1, Supplementary Table 1). Flower numbers were initially oscillating in synchrony between the treatment groups, however after the CO2 treatments began the eCO2 treatment desynchronised with both the aCO2 and undisturbed plots, producing more flowers than the undisturbed plots during the 2018 failed crop (Fig. 3, eCO2 / undisturbed: P < 0.05); although not significantly more than ambient (Fig. 3, eCO2 / aCO2: P = 0.86). Flower development uses assimilate stored in the trunk or roots (Hoch et al. 2013). Hence, flower counts may not have been expected to respond to the initial season under elevated CO2, but greater net photosynthesis under elevated CO2 (Gardner et al. 2022b) may have refilled stored reserves more quickly later in 2017. The higher-than-expected number of flowers in the eCO2 plot during 2018 may be evidence of this (Fig. 3). After 2018, flower numbers broadly returned to synchrony among the treatments. It may be that trees under elevated CO2 acclimatised to the increased CO2 and/or switched the additional carbon resource from reproduction into maintenance and growth, as evidenced by the higher leaf mass per unit area (Gardner et al. 2022c) found in the eCO2 cf. aCO2 arrays at the site.
The undisturbed treatment produced the most mature acorns on average during the recorded period of CO2 enrichment, with no difference between eCO2 and aCO2 (Fig. 2, Supplementary Table 1). To have found no increase in mature acorn number for eCO2 over aCO2 is surprising as it disagrees with the results from an open-top chamber study of scrub oaks (Stiling et al. 2004) and for seeds of Pinus taeda L. (LaDeau and Clark 2001; Way et al. 2010). There were, however, higher counts of empty cups observed after CO2 enrichment began and when all evidence of acorns is combined an increase is detected within the eCO2 treatment (Figs. 2 and 3). This suggests an increase in acorn production resultant from elevated CO2 that is being obscured by greater acorn predation rates. Seed predators favour larger, more mature acorns (Gómez 2004). Whilst efforts are made to control the numbers of seed predators at the BIFoR FACE site (Bradwell 2022), it is clear from camera trapping that they remain present. Moreover, the litter traps used were not sealed. Hence, one likely explanation is that post-dispersal acorn predation in the litter traps reduced the counts of mature acorns, leaving behind empty cups and immature acorns; as such, counts of empty cups, immature acorns, and the composite group all acorn evidence are potentially more reliable indicators of the impact of CO2 on acorn production in this study. The importance of predation in determining the fate of reproductive material under eCO2 illustrates the importance of process-permitting experimental designs such as FACE in contrast to more closed systems.
There were significant interactions between treatment and year for immature acorns, empty cups, and all acorn evidence, suggesting these groups are increasing after CO2 enrichment began (Table 1; Figs. 2 and 3). At the start of the experiment the number of empty cups, immature acorns and all evidence of acorns were lowest in the eCO2 plots, but post-treatment application the eCO2 plots were producing the highest counts of these reproductive material categories (Table 1; Figs. 2 and 3). Treatments were assigned to FACE arrays to pair aCO2 and eCO2 treatments as geographically close as practicable, but the variation observed in the pre-enrichment period suggests that other underlying differences remain between the aCO2 and eCO2 treatment arrays. The increase in these groups of reproductive material over this period might indicate an increase in post-fertilisation production of acorns under elevated CO2. That the number of mature acorns does not increase in line with this might indicate inadequate nutrient supply to support acorn development to maturity. The numbers of immature acorns (i.e., early-aborting acorns) were greatest in the eCO2 treatment for 2020 and 2021 (Fig. 3). There is, however, no evidence for nutrient limitation in leaf composition at BIFoR FACE (Gardner et al. 2022c). Moreover, post-hoc pairwise comparison showed the higher immature acorn counts in the eCO2 plots in these years was not statistically significant, however; as the experiment continues it is worth monitoring this putative trend. If elevated CO2 were to increase the numbers of immature acorns produced each year this might damage the management of Q. robur by negating the seed predator defence of masting (Bogdziewicz et al. 2020). The higher numbers of immature acorns could provide a bridging effect between mast years, maintaining consistently higher seed predator populations that would eat higher proportions of mature acorns during the mast year.
Attacks from pre-dispersal predators, in this case exclusively oak knopper galls (Andricus quercuscalicis Burgsdorf), showed no significant effects of the plots on galls in the two years before the CO2 treatments were applied (Fig. 2), but thereafter galls were more common in the undisturbed and eCO2 treatments (Table 1 and Fig. 2). The greater numbers of mature acorns in the undisturbed plot might explain why more galls were found (i.e., with more acorns one may expect more galls). However, the eCO2 treatment had the lowest mature acorn count on average and yet the number of galls was three times higher than the aCO2 treatment (Fig. 2). If one assumes that each gall could have developed into a mature acorn if it had not been attacked, then eCO2 would have produced more acorns than aCO2 on average: galls plus mature acorns were 3.43 per m2 of litter trap under eCO2 and 2.15 under aCO2; and this difference would be yet greater if the numbers of immature acorn were also added (see Fig. 2). The higher attack rate by galls observed may thus have contributed to more acorns failing to reach maturity in the eCO2 plots. These results suggest that the presence of more developing acorns in elevated CO2 may be of greater benefit to seed predators than to the regeneration of the trees themselves, as noted by Bogdziewicz et al. (2020). Moreover, higher growth under CO2 may result in higher mortality rates in mature trees (Büntgen et al. 2019; Brienen et al. 2020). And so, the potential benefits from future increase in CO2 may, conversely, result in fewer juvenile trees with shorter lifespans.
Elevated CO2 has been found to increase herbivore numbers in some years (Kampichler et al. 2008), but this effect may be specific to species and functional groups because other species have shown the opposite result (Hall et al. 2005; Roberts et al. 2022). In this study the difference in the numbers of galls in the periods before and after enrichment was not statistically significant under any treatment (Fig. 2), agreeing with previous research at the site into herbivory per unit area of leaf on Q. robur (Roberts et al. 2022). Pre-enrichment counts suggest that the eCO2 plots were already experiencing higher pre-dispersal seed predation, with two years of data giving insufficient power to pick up on this difference statistically.
Studies of this type are exceedingly rare due to the resources required to set up such large, long-duration experiments. Moreover, the requirement to build such investigations around long-established trees in mature woodlands means that the experimental sites cannot be as homogeneous as with, for example, experimental grounds with annual plants. Nonetheless, this study provides strong evidence of bottlenecks to fruit production and the ultimate fate of fruit in old temperate forest subject to predation by granivorous vertebrates. Our results suggest that the effects of elevated CO2 on acorn production increase over time (Fig. 3, ‘all evidence’ panel) and so may be cumulative, especially as masting works on multi-year cycles. Hence it will be important to carry on the study for some years to determine if more pronounced effects emerge over time.
Conclusions
Using FACE to increase the atmospheric CO2 concentration by 150 μL L−1 in old, temperate deciduous woodland, had no significant direct effect on the phenomenon of masting in Quercus robur L., nor on the numbers of either enlarged cups or mature acorns, but it did increase significantly the numbers of empty cups, immature acorns, and all evidence of acorns. All categories of reproductive material were affected by year, with Q. robur at this site conforming to fruit maturation masting behaviour. We suggest that elevated CO2 increased the initial numbers of acorns developing, but this was not reflected by the mature acorn counts due to (1) higher load of pre-dispersal seed predation in the elevated CO2 plots, and (2) post-dispersal seed predators taking mature acorns. We have no evidence currently for a third possible explanation of nutrient limitations under eCO2 preventing full acorn development. The number of immature acorns also increased under eCO2 from being the lowest of the three treatments in the early years to the highest by the final two studied, suggesting that the effects of elevated CO2 may be cumulative—which requires further study. Flower number was also affected by the interaction of CO2 treatment with year, suggesting elevated CO2 may affect the cycle of resource expenditure and storage, but in this regard the trees appeared to acclimatise to this increase in CO2 quickly.
References
Ainsworth EA, Long SP (2005) What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytol 165:351–372. https://doi.org/10.1111/j.1469-8137.2004.01224.x
Ainsworth EA, Long SP (2021) 30 years of free-air carbon dioxide enrichment (FACE): What have we learned about future crop productivity and its potential for adaptation? Global Change Biol 27:27–49. https://doi.org/10.1111/gcb.15375
Akaike H (1998) Information theory and an extension of the maximum likelihood principle. In: Parzen E, Tanabe K, Kitagawa G (eds) Selected Papers of Hirotugu Akaike. Springer, pp 199–213.
Alam SM (1999) Nutrient uptake by plants under stress conditions. In: Pessaraki M (ed) Handbook of Plant and Crop Stress. Marcel Dekker, New York, pp 285–314
Allen RB, Millard P, Richardson SJ (2017) A Resource Centric View of Climate and Mast Seeding in Trees. In: Cánovas FM, Lüttge U, Matyssek R (eds.), Progress in Botany. Vol. 79. Springer International Publishing, Cham, pp 233–268. https://doi.org/10.1007/124_2017_8
Askeyev O, Tishin D, Sparks T, Askeyev I (2005) The effect of climate on the phenology, acorn crop and radial increment of pedunculate oak (Quercus robur) in the middle Volga region, Tatarstan, Russia. Int J Biometeorol 49:262–266. https://doi.org/10.1007/s00484-004-0233-3
Boavida LC, Silva JP, Feijó JA (2001) Sexual reproduction in the cork oak (Quercus suber L.). II. Crossing intra-and interspecific barriers. Sex Plant Reprod 14:143–152. https://doi.org/10.1007/s004970100100
Bogdziewicz M, Szymkowiak J, Fernandez-Martinez M, Penuelas J, Espelta JM (2019) The effects of local climate on the correlation between weather and seed production differ in two species with contrasting masting habit. Agric for Meteorol 268:109–115. https://doi.org/10.1016/j.agrformet.2019.01.016
Bogdziewicz M, Kelly D, Thomas PA, Lageard JG, Hacket-Pain A (2020) Climate warming disrupts mast seeding and its fitness benefits in European beech. Nat Plants 6:88–94
Bole D (2022) Addressing the possible shortfall of oak for the 2022/23 planting season. URL https://forestrycommission.blog.gov.uk/2022/07/21/addressing-the-possible-shortfall-of-oak-for-the-2022-23-planting-season/ [accessed on 13 July 2023]
Bradwell AR (2022) Norbury Park: An estate tackling climate change. Norbury Park Estate. ISBN: 978-1-5272-9734-0. Available from office@harbourneoffice.co.uk
Brienen RJW, Caldwell L, Duchesne L, Voelker S, Barichivich J, Baliva M, Ceccantini G, Di Filippo A, Helama S, Locosselli GM, Lopez L, Piovesan G, Scöngart J, Villalba R, Gloor E (2020) Forest carbon sink neutralized by pervasive growth-lifespan trade-offs. Nat Commun 11:4241. https://doi.org/10.1038/s41467-020-17966-z
Büntgen U, Krusic PJ, Piermattei A, Coomes DA, Esper J, Myglan VS, Kirdyanov AV, Camarero JJ, Crivellaro A, Körner C (2019) Limited capacity of tree growth to mitigate the global greenhouse effect under predicted warming. Nat Commun 10:2171. https://doi.org/10.1038/s41467-019-10174-4
Canelo T, Gaytán Á, González-Bornay G, Bonal R (2018) Seed loss before seed predation: experimental evidence of the negative effects of leaf feeding insects on acorn production. Integr Zool 13:238–250. https://doi.org/10.1111/1749-4877.12292
Darbah JNT, Kubiske ME, Nelson N, Oksanen E, Vapaavuori E, Karnosky DF (2008) Effects of decadal exposure to interacting elevated CO2 and/or O3 on paper birch (Betula papyrifera) reproduction. Environ Pollut 155:446–452. https://doi.org/10.1016/j.envpol.2008.01.033
De Graaff MA, Van Groenigen KJ, Six J, Hungate B, van Kessel C (2006) Interactions between plant growth and soil nutrient cycling under elevated CO2: a meta-analysis. Global Change Biol 12:2077–2091. https://doi.org/10.1111/j.1365-2486.2006.01240.x
Dickson R, Tomlinson P (1996) Oak growth, development and carbon metabolism in response to water stress. Ann for Sci 53:181–196. https://doi.org/10.1051/forest:19960202
US DOE (2020) US Department of energy free-air CO2 enrichment experiments: FACE results, lessons, and legacy. DOE/SC–0202. U.S. Department of Energy Office of Science. https://doi.org/10.2172/1615612.
Drake BG, Gonzàlez-Meler MA, Long SP (1997) More efficient plants: a consequence of rising atmospheric CO2? Annu Rev Plant Physiol Plant Mol Biol 48:609–639. https://doi.org/10.1146/annurev.arplant.48.1.609
Espelta JM, Cortés P, Molowny-Horas R, Sánchez-Humanes B, Retana J (2008) Masting mediated by summer drought reduces acorn predation in mediterranean oak forests. Ecology 89:805–817. https://doi.org/10.1890/07-0217.1
Flannigan MD, Stocks BJ, Wotton BM (2000) Climate change and forest fires. Sci Tot Environ 262:221–229
Fleurot E, Lobry JR, Boulanger V, Debias F, Mermet-Bouvier C, Caignard T, Delzon S, Bel-Venner MC, Venner S (2023) Oak masting drivers vary between populations depending on their climatic environments. Curr Biol 33:1117-1124.e4. https://doi.org/10.1016/j.cub.2023.01.034
Gardner A, Ellsworth DS, Crous KY, Pritchard J, MacKenzie AR (2022a) Is photosynthetic enhancement sustained through three years of elevated CO2 exposure in 175-year-old Quercus robur? Tree Physiol 42:130–144. https://doi.org/10.1093/treephys/tpab090
Gardner A, Ellsworth DS, Pritchard J, MacKenzie AR (2022b) Are chlorophyll concentrations and nitrogen across the vertical canopy profile affected by elevated CO2 in mature Quercus trees? Trees 36:1797–1809. https://doi.org/10.1007/s00468-022-02328-7
Gardner A, Jiang M, Ellsworth DS, MacKenzie AR, Pritchard J, Bader MKF, Barton C, Bernacchi C, Calfapietra C, Crous KY, Dusenge ME, Gimeno TE, Hall M, Lamba S, Leuzinger S, Uddling J, Warren J, Wallin G, Medlyn BE (2022c) Optimal stomatal theory predicts CO2 responses of stomatal conductance in both gymnosperm and angiosperm trees. New Phytol 237(4):1229–1241. https://doi.org/10.1111/nph.18618
Gómez JM (2004) Bigger is not always better: conflicting selective pressures on seed size in Quercus ilex. Evolution 58:71–80. https://doi.org/10.1111/j.0014-3820.2004.tb01574.x
Hacket-Pain A (2021) Masting. Curr Biol 31:R884–R885. https://doi.org/10.1016/j.cub.2021.06.007
Hall MC, Stiling P, Moon DC, Drake BG, Hunter MD (2005) Effects of elevated CO2 on foliar quality and herbivore damage in a scrub oak ecosystem. J Chem Ecol 31:267–286. https://doi.org/10.1007/s10886-005-1340-2
Hart KM, Curioni G, Blaen P, Harper NJ, Miles P, Lewin K, Nagy J, Bannister EJ, Cai XM, Thomas RM, Krause S, Tausz M, MacKenzie AR (2019) Characteristics of free air carbon dioxide enrichment of a northern temperate mature forest. Global Change Biol 26:1023–1037. https://doi.org/10.1111/gcb.14786
Hendrey GR, Ellsworth DS, Lewin KF, Nagy J (1999) A free-air enrichment system for exposing tall forest vegetation to elevated atmospheric CO2. Global Change Biol 5:293–309. https://doi.org/10.1046/j.1365-2486.1999.00228.x
Hoch G, Siegwolf RT, Keel SG, Körner C, Han Q (2013) Fruit production in three masting tree species does not rely on stored carbon reserves. Oecol 171:653–662
Ibáñez I, Clark JS, Dietze MC, Feeley K, Hersh M, LaDeau S, McBride A, Welch NE, Wolosin MS (2006) Predicting biodiversity change: Outside the climate envelope, beyond the species–area curve. Ecology 87:1896–1906. https://doi.org/10.1890/0012-9658(2006)87[1896:PBCOTC]2.0.CO;2
IPCC (2023) Summary for Policymakers. In: Climate Change 2023: Synthesis Report. Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change [Core Writing Team, H. Lee and J. Romero (eds.)]. IPCC, Geneva, Switzerland, pp. 1–34, https://doi.org/10.59327/IPCC/AR6-9789291691647.001
Isagi Y, Sugimura K, Sumida A, Ito H (1997) How does masting happen and synchronize? J Theor Biol 187:231–239. https://doi.org/10.1006/jtbi.1997.0442
Jablonski LM, Wang X, Curtis PS (2002) Plant reproduction under elevated CO2 conditions: a meta-analysis of reports on 79 crop and wild species. New Phytol 156:9–26. https://doi.org/10.1046/j.1469-8137.2002.00494.x
Kampichler C, Teschner M, Klein S, Körner C (2008) Effects of 4 years of CO2 enrichment on the abundance of leaf-galls and leaf-mines in mature oaks. Acta Oecol 34:139–146. https://doi.org/10.1016/j.actao.2008.05.006
Kelly D (1994) The evolutionary ecology of mast seeding. Trends Ecol Evol 9:465–470. https://doi.org/10.1016/0169-5347(94)90310-7
Khaine I, Woo SY (2015) An overview of interrelationship between climate change and forests. For Sci Technol 11:11–18. https://doi.org/10.1080/21580103.2014.932718
Kimball BA, Kobayashi K, Bindi M (2002) Responses of agricultural crops to free-air CO2 enrichment. Adv Agron 77:293–368. https://doi.org/10.1016/S0065-2113(02)77017-X
Koenig WD, Knops JMH (2005) The mystery of masting in trees: Some trees reproduce synchronously over large areas, with widespread ecological effects, but how and why? Am Sci 93:340–347. http://www.jstor.com/stable/27858609
Komatsu H, Katayama A, Hirose S, Kume A, Higashi N, Ogawa S, Otsuki K (2007) Reduction in soil water availability and tree transpiration in a forest with pedestrian trampling. AgricFor Meteorol 146:107–114. https://doi.org/10.1016/j.agrformet.2007.04.014
LaDeau SL, Clark JS (2001) Rising CO2 levels and the fecundity of forest trees. Science 292:95–98. https://doi.org/10.1126/science.1057547
Langsrud Ø (2003) ANOVA for unbalanced data: Use Type II instead of Type III sums of square. Stat Comput 13:163–167. https://doi.org/10.1023/A:1023260610025
Long SP, Ainsworth EA, Rogers A, Ort DR (2004) Rising atmospheric carbon dioxide: plants FACE the future. Annu Rev Plant Biol 55:591–628. https://doi.org/10.1146/annurev.arplant.55.031903.141610
MacKenzie AR, Krause S, Hart KM, Thomas RM, Blaen PJ, Hamilton RL, Curioni G, Quick SE, Kourmouli A, Hannah DM, Comer-Warner SA, Brekenfeld N, Ullah S, Press MC (2021) BIFoR FACE: water–soil–vegetation–atmosphere data from a temperate deciduous forest catchment, including under elevated CO2. Hydrolo Process 35:e14096. https://doi.org/10.1002/hyp.14096
Martínez-Baroja L, Pérez-Camacho L, Villar-Salvador P, Rebollo S, Quiles P, Gómez-Sánchez D, Molina-Morales M, Leverkus AB, Castro J, Rey-Benayas JM (2019) Massive and effective acorn dispersal into agroforestry systems by an overlooked vector, the Eurasian magpie (Pica pica). Ecosphere 10:e02989. https://doi.org/10.1002/ecs2.2989
Mayoral C, Ioni S, Luna E, Crowley L, Hayward S, Sadler JP, Mackenzie AR (2023) Elevated CO2 does not improve seedling performance in a naturally regenerated oak woodland exposed to biotic stressors. Front for Glob Change 6:1278409. https://doi.org/10.3389/ffgc.2023.1278409
Obeso JR (2002) The costs of reproduction in plants. New Phytol 155:321–348. https://doi.org/10.1046/j.1469-8137.2002.00477.x
Palacio S, Hoch G, Sala A, Körner C, Millard P (2014) Does carbon storage limit tree growth? New Phytol 201:1096–1100. https://doi.org/10.1111/nph.12602
Pau S, Okamoto DK, Calderón O, Wright SJ (2018) Long-term increases in tropical flowering activity across growth forms in response to rising CO2 and climate change. Global Change Biol 24:2105–2116. https://doi.org/10.1111/gcb.14004
Pearse IS, Koenig WD, Kelly D (2016) Mechanisms of mast seeding: resources, weather, cues, and selection. New Phytol 212:546–562. https://doi.org/10.1111/nph.14114
Poorter H, Navas ML (2003) Plant growth and competition at elevated CO2: on winners, losers and functional groups. New Phytol 157:175–198. https://doi.org/10.1046/j.1469-8137.2003.00680.x
Pritchard SG, Rogers HH, Prior SA, Peterson CM (1999) Elevated CO2 and plant structure: a review. Global Change Biol 5:807–837. https://doi.org/10.1046/j.1365-2486.1999.00268.x
R Core Team (2021) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/
Roberts AJ, Crowley LM, Sadler JP, Nguyen TT, Gardner AM, Hayward SA, Metcalfe DB (2022) Effects of elevated atmospheric CO2 concentration on insect herbivory and nutrient fluxes in a mature temperate forest. Forests 13:998. https://doi.org/10.3390/f13070998
Ruehr S, Keenan TF, Williams C, Zhou Y, Lu X, Bastos A, Canadell JG, Prentice IC, Sitch S, Terrer C (2023) Evidence and attribution of the enhanced land carbon sink. Nat Rev Earth Environ 4:518–534. https://doi.org/10.1038/s43017-023-00456-3
Sever K, Bogdan S, Škvorc Ž (2023) Response of photosynthesis, growth, and acorn mass of pedunculate oak to different levels of nitrogen in wet and dry growing seasons. J for Res 34:167–176. https://doi.org/10.1007/s11676-022-01505-1
Skopp J, Jawson MD, Doran JW (1990) Steady-state aerobic microbial activity as a function of soil water content. Soil Sci Soc Am J 54:1619–1625. https://doi.org/10.2136/sssaj1990.03615995005400060018x
Stiling P, Moon D, Hymus G, Drake B (2004) Differential effects of elevated CO2 on acorn density, weight, germination, and predation among three oak species in a scrub-oak forest. Global Change Biol 10:228–232. https://doi.org/10.1111/j.1365-2486.2004.00728.x
Sturrock RN, Frankel SJ, Brown AV, Hennon PE, Kliejunas JT, Lewis KJ, Worrall JJ, Woods AJ (2011) Climate change and forest diseases. Plant Pathol 60:133–149. https://doi.org/10.1111/j.1365-3059.2010.02406.x
Wand SJ, Midgley GF, Jones MH, Curtis PS (1999) Responses of wild C4 and C3 grass (Poaceae) species to elevated atmospheric CO2 concentration: a meta-analytic test of current theories and perceptions. Global Change Biol 5:723–741. https://doi.org/10.1046/j.1365-2486.1999.00265.x
Way DA, Ladeau SL, McCarthy HR, Clark JS, Oren RAM, Finzi AC, Jackson RB (2010) Greater seed production in elevated CO2 is not accompanied by reduced seed quality in Pinus taeda L. Global Change Biol 16:1046–1056. https://doi.org/10.1111/j.1365-2486.2009.02007.x
Wesołowski T, Rowiński P, Maziarz M (2015) Interannual variation in tree seed production in a primeval temperate forest: Does masting prevail? Eur J for Res 134:99–112. https://doi.org/10.1007/s10342-014-0836-0
Wheeler TR, Daymond AJ, Morrison JIL, Ellis RH, Hadley P (2004) Acclimation of photosynthesis to elevated CO2 in onion (Allium cepa) grown at a range of temperatures. Ann App Biol 144:103–111. https://doi.org/10.1111/j.1744-7348.2004.tb00322.x
Zhu Z, Piao S, Myneni R, Huang M, Zeng Z, Canadell JG, Ciais P, Sitch S, Friedlingstein P, Arneth A, Cao C, Cheng L, Kato E, Koven C, Li Y, Lian X, Liu Y, Liu R, Mao J, Pan Y, Peng S, Peñuelas J, Poulter B, Pugh TAM, Stocker BD, Viovy N, Wang X, Wang Y, Xiao Z, Yang H, Zaehle S, Zeng N (2016) Greening of the Earth and its drivers. Nat Clim Chang 6:791–795. https://doi.org/10.1038/nclimate3004
Acknowledgements
We thank Giulio Curioni, Gael Denny, and Deanne Brettle for their work to collect, process, and archive leaf litter material from the BIFoR FACE arrays. We thank Future Trees Trust, The Patsy Wood Trust, Scottish Forestry Trust, Aitchinson Tait Trust, and Action Oak for funding RM’s PhD studies. CM and ARMK acknowledge support from the UK Natural Environment Research Council (NE/S015833/1 (QUINTUS)). The BIFoR-FACE facility acknowledges generous underpinning support from the JABBS Trust, Norbury Park Estate, The John Horseman Trust, Ecological Continuity Trust, and the University of Birmingham. Access to BIFoR Core Data was funded by Royal Society University Research Fellowship URF\R1\191326.
Funding
RM’s PhD study was supported by Future Trees Trust, The Patsy Wood Trust, Scottish Forestry Trust, Aitchinson Tait Trust, and Action Oak for funding. CM and ARMK acknowledge support from the UK Natural Environment Research Council (NE/S015833/1 (QUINTUS)). The BIFoR-FACE facility acknowledges generous underpinning support from the JABBS Trust, Norbury Park Estate, The John Horseman Trust, Ecological Continuity Trust, and the University of Birmingham. Access to BIFoR Core Data was funded by Royal Society University Research Fellowship URF\R1\191326.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The online version is available at http://link.springer.com.
Corresponding editor: Yanbo Hu.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
McClory, R., Ellis, R.H., Lukac, M. et al. Carbon dioxide enrichment affected flower numbers transiently and increased successful post-pollination development stably but without altering final acorn production in mature pedunculate oak (Quercus robur L.). J. For. Res. 35, 73 (2024). https://doi.org/10.1007/s11676-024-01724-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11676-024-01724-8