Abstract
Subri River Forest Reserve (SR) is the most extensive forest area in Ghana with an accompanying rich floral species. Over the years, logging from both legally prescribed and illegal operations remain the predominant forest disturbance in SR. Gap creation following logging is crucial in determining tree species composition and diversity. Hence, the study evaluated the composition and diversity of naturally regenerated tree species in logging gaps of different sizes and, again examined the roles of these tree species in fulfilling the economic and ecological agenda of sustainable forest management after logging in SR. Twelve gaps were randomly selected: 4 each were grouped into small size (≤ 200 m2), medium size (201–300 m2), and large size (≥ 300 m2). Data were gathered from 1 m2 circular area at gap centres and repeatedly inside 1 m width strip along 20 m individual N-S-E-W transects. Species diversity differed significantly between gap sizes. Higher diversity indices were measured in large size gaps. Gap sizes shared similar species. There were significant differences among various height groupings of tree species across all three gap sizes. Pioneers preferred medium to large size gaps, while shade-tolerant tree species preferred small size gaps for their abundance. Vulnerable and Lower Risk Near Threatened tree species under Conservation Status and, Premium and Commercial tree species under Utilisation Status preferred small size gaps for their proliferation and conservation. Therefore, we recommend the single tree-based selective logging for ensuring creations of small to medium size (200–300 m2) gaps through adjustments to the logging permit process, revision of Allocation Quota Permit, strict adherence to the 40-year polycyclic selection system, along with more dedicated enforcement and monitoring. Changes along these protocols would tremendously facilitate natural regeneration of different suites of timber species resulting in the improvement of the overall biodiversity conservation associated with the forest, more sustainable forest harvests and more income to those who receive permits.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Logging has been one of the primary contributory factors of deforestation within the Tropical African forests which form the Guineo-Congolian region serving as the habitat for more than 1000 endemic plant species (Parren and Graaf 1995). In West Africa, logging operations began in the sixteenth century when Lophira alata species was first exploited and exported to the United Kingdom (Wilks et al. 1985). By the end of the nineteenth century, French companies actively got involved in the early commercial forest exploitation where they felled and exported Khaya and Entandrophragma species. Later, Liberia, Cote d'Ivoire, and Ghana attained “important timber exporting countries status” in the lowland moist forest zone (Parren and Graaf 1995).
Ghana's forests are characterized by abundant complexity of floristic composition with an incredible biodiversity (Agyeman et al. 1999) containing about 730 tree species with 106 megaphanerophytes species usable for timber production (Hall and Swaine 1981). Forests of these rich floristic attributes are largely situated within the High Forest Zone (HFZ) of Ghana-an ecological zone with an outstanding repository of biodiversity (MLNR 2012). Out of 266 forest reserves found within the HFZ (MLNR 2012). Subri River Forest Reserve (SR) is the most extensive reserve with exceptional plant diversity and highly diversified forest ecosystems that provide conducive natural habitats for over 200 tree species of diverse economic and ecological importance. Due to the rich floristic nature of SR, logging operations continue as an eminent form of forest disturbance since 1978 (Abu-Juam and Hawthorne 1994; Forestry Commission of Ghana 2002). Thus, the delineation of SR areas into valuable and vulnerable sites is based on species conservation priority (Abu-Juam and Hawthorne 1994; Bach 1999), hold/legacy of diversified tree species present, and the intensity or frequency of logging operations. Generally, in Ghana, logging from the selection system (Hawthorne 1995; Hawthorne et al. 2012) or illegal logging activities or both sources (Herrmann 2011) remain the predominant forest disturbance that defines forest structure, tree species composition and diversity, and most importantly, determines the sustainability of natural regeneration in gaps (Hammond et al. 2021).
Natural regeneration in gaps is often considered an associated positive effect of logging disturbance. Many papers have expressed it in lowland evergreen rainforest, Amazonia forests (Darrigo et al. 2016; De Carvalho et al. 2017), tropical moist semi-deciduous forests (Hawthorne 1995; Duah-Gyamfi et al. 2014; Hammond et al. 2021), dry Sal forests (Sapkota and Oden 2009), tropical dry semi-deciduous forest (Appiah 2013), temperate Asia secondary forest (Lu 2015), temperate mixed European forests (Nagel et al. 2010; Hammond et al. 2020), etc. Logging usually creates gap openings with varying sizes within the canopy cover. These logging gaps provide heterogenous microclimatic conditions such as light, moisture, and temperature (Yamamoto 2000; Martins and Rodrigues 2005; Duah-Gyamfi et al. 2014; Hammond and Pokorný 2020b; 2020c), which are essential for a wide range of natural regeneration of tree species with different levels of shade tolerance mechanisms (Nagel et al. 2010; Hammond and Pokorný 2020a). However, physical gap characteristics have a strong influence on the overall regeneration process (Martins and Rodrigues 2005; Sapkota and Oden 2009), particularly the gap size feature which is often used as a heterogeneity indicator of microclimatic conditions and resource sequestration within canopy openings (Agyeman et al. 1999; McCarthy 2001). Variation of gap size is directly linked with the intensity of logging disturbance (De Carvalho et al. 2017), alongside tree size, crown dimensions, and single or multiple tree falls (McCarthy 2001; Muscolo et al. 2014; Hammond and Pokorný 2020b). Previous studies suggest gap size as the most crucial determining factor in tree species composition in gap regeneration management (Nagel et al. 2010; Hammond et al. 2020) because large gaps determine the maintenance of pioneer/shade-intolerant tree species whilst small gaps are a beneficial stimulator for shade-tolerant tree species regeneration (Martins and Rodrigues 2005; Lu et al. 2015). Gaps largely promote diversity of naturally regenerated tree species through the provision of yielding microsites for the coexistence of tree species with different light requirements. Therefore, gap regeneration can be used as an effective forest management tool in restoring the establishment of different tree species with varied light-specific characteristics following logging (Muscolo et al. 2014; Hammond and Pokorný 2020a).
However, only a few studies have been conducted in tropical forests to ascertain the imperative effects of gaps of different sizes on the natural regeneration of tree species in terms of their composition and diversity after logging, especially in Ghana. Therefore, there is the need to broaden the knowledge scope of gap-species dynamics, particularly on post-logging silvicultural practices to predict species composition and diversity of the future stand structure. To precisely describe the importance of SR in Ghana, specifically in terms of economic benefits and environmental protection. Hence, the objectives of the study were (1) To estimate the sizes of formed gaps at SR following logging; (2) To evaluate and compare naturally regenerated tree species composition, diversity and growth dynamics in small size, medium size and large size gaps and finally; and, (3) To examine the roles of natural regeneration across gap sizes in fulfilling economic and ecological aspects of sustainable forest management after logging at SR.
Materials and methods
Study area
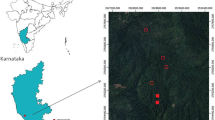
The study area, SR (Fig. 1) is in the southwest of Ghana, precisely located between latitudes 5°05′ − 5°30′ N and longitudes 1°35′ − 1°55′ W with an area coverage of over 50,000 ha. Due to SR's expansive size, it is further categorised into Daboase, Ateiku, Wassa Nkran and Benso ranges. The reserve has 483 compartments of approximately 128 ha each (800 × 1600 m). SR has a rolling topography which lies between 60 and 125 m, a.s.l. elevation under a prevailing tropical humid climate with mean annual relative air humidity of 85% throughout the year. The mean annual air temperature and precipitation range between 23–29 °C and 1500–2150 mm, respectively. Ferruginised rock is the predominant underlying bedrock with forest oxysols (same as the oxisols in the US soil classification system) and lithosols (Diame 2007) as the most dominant soil groups according to the Ghana soil classification system (Brammer 1962). Also, the reserve is drained by the ‘Subri’ river from which its name was derived.
The study area is broadly classified as an evergreen forest type. Nonetheless, forest areas are further characterized as Moist (humid) or Wet (wetter) evergreen forest subtypes according to their site-specific ecological conditions. Celtis-Triplochiton (Cannabaceae-Malvaceae) association is prominently found from the north to the west side while Lophira-Triplochiton (Ochnaceae-Malvaceae) association is more pronounced in the south (Taylor 1960). However, Cynometra spp. (Caesalpinioideae), Lophira spp. and Heritiera spp. (Malvaceae) are more present in the wetter areas while Celtis spp. and Triplochiton spp. are primarily found in the drier areas within the reserve (Taylor 1960). For every 3,970 trees with over 10 cm dbh (stem diameter at the breast height), at least a minimum of 90 tree species would be observed (Taylor 1960). The average canopy height of tree species is about 30 m. In the upper canopy cohort, a few non-native deciduous tree species are present. Usually, Lophira alata, Piptadeniastrum africanum, Parkia bicolor and Tarrietia utilis form the upper canopy cohorts, while Diospyros sanza-minka, Funtumia africana, Allanblackia parviflora and Cola spp. form the lower canopy cohorts. Dacryodes klaineana species are predominant between upper and lower canopy layers (Diame 2007). By ecology, several tree species often find their optimal niches and abundances within this forest reserve. Hence, SR is classified under Category IV (habitat/species management area) of IUCN (International Union for Conservation of Nature) protected area categories (MLNR 2016). Currently, the proportion abundance of native commercial Entandophragma cylindricum and Lovoa trichilioides species in SR are heavily threatened. IUCN (2004) the former tree species is considered ‘Vulnerable’ while the latter tree species is deemed as ‘Least Concern’.
Natural regeneration, canopy tree management strategies, selection, seed tree and shelterwood systems, and coppicing (mainly with P. africanum species) are the leading engaged silviculture practices in SR. Nevertheless, Taungya (i.e., a forestry system that involves inter-planting trees with food crops), seed orchid and admitted farming are used to manage degraded areas within the reserve.
Data collection
Gap identification, size estimation and classification
Due to the slight variations in the climatic rainfall pattern across different areas within SR, forest site conditions vary across different areas within the reserve. Based on this, compartments with similar site conditions and forest types at the northern part of SR where low to high intensity of logging disturbances by legal Timber Utilisation Contract (TUC) holders had been completed at least eight months before were selected. Specifically, two compartments each from the northwest and northeast sites were considered for data sampling. In total, twelve artificial gaps of different sizes were selected from northwest compartments 18 and 19 and northeast compartments 41 and 43 at the study area (Fig. 1). A total number of 4 small size gaps, two each were randomly selected from compartments 18 and 19, respectively. The justification was that these compartments were logged in 2017 and with time, the total gap area formed from logging had a clear decreasing trend with many gaps almost closed (Fox 2000), leaving only a few gaps with viable openings. However, 4 gaps each classified as medium size and large size were randomly selected from compartments 41 and 43, respectively, because more and larger canopy openings were present there. These gaps were formed in 2018, and they showed either slight or no changes in gap dynamics. Besides, selection of gaps was based on the following criteria: (1) suitable gap microsite conditions such as: detection of moderate effects of logging (i.e., absence of widespread skid trails; minimum soil surface disturbances; controlled presence of offcuts) and (2) suitable “ecological” conditions of the overall stand such as: slight indications of natural disturbances. Gaps that could not meet all criteria were exempted from the data survey.
Data were gathered in January 2019. Garmin (62 s) handheld portable Global Positioning System (GPS) was used to obtain geographical points from eight different spots (0°, 45°, 90°, 135°, 180°, 225°, 270°, and 315°) around the borders of each gap. Coordinates were then subjected to 2019 AutoCAD software for the generation of gap sizes. Subsequently, gap sizes ranging between 25 and 200 m2 were classified as small size, 201–300 m2 as medium size and over 301 m2 as large size (Table 1).
Experimental gap design
At each gap site, the gap centre was first identified and marked as a reference or starting point, then 4 sampling 20 m long transects in the North, South, East and West (N-S-E-W) directions were marked out. Thereafter, at gap centre, 1-m2 circular sampling area (i.e., radius of 56 cm) was delineated. Next, 1-m width strip along individual N-S-E-W transects were demarcated within each selected gap (Fig. 2).
Identification of native commercial tree species and data survey
From each selected gap, all naturally regenerated native tree species were identified, followed by measurements of their respective heights and occurrence frequencies within demarcated sampling areas of gap centre and N-S-E-W transects. This was repeated for all studied gaps. Data surveys were undertaken appropriately with assistance from a local botanist, experienced Forest Guards and Forestry Range Supervisors. To minimize errors, two botanical books (Hawthorne and Gyakari 2006; Hawthorne and Jongkind 2006) were concurrently referred to. For communicating findings more effectively, tree species were classified into homogeneous groups to impose a degree of simplification that would explicitly reveal general patterns and facilitate predictions about gap regeneration and its related composition following logging disturbances. In the light of this, natural regeneration tree species were further categorised into the various groupings according to; Height growth (Table 6), Guilds (functional groups based on shade tolerance mechanisms) (Table 2) (Hammond et al. 2021), Conservation Status-fundamentally based on conservation state of tree species; life-long exploitation attributes and intensity, and sustainability (Table 2) and Utilisation Status-primarily based on how long a tree species has been exported; wood quality; and demand on the export market (Table 2) according to Oteng-Amoako (2006) descriptions.
Data analysis
The Paleontological Statistics software package, (PAST 3.24 version) (Hammer et al. 2001) was used to perform the analyses of the evaluated biodiversity indices. Six diversity indices (Eqs. 1–6) were appropriately considered because of their pertinence to the study goals.
General Linear Multivariate Analysis was performed using TIBCO STATISTICA software programme (13.4.0.14 version) followed by a post hoc Tukey’s HSD test to compare significant differences of the various evaluated diversity indices, guild categories, and height growth groupings between gaps of different sizes (n = 4 per each gap size) at p < 0.05 significance level. Prior to this, all analyzed data set were ensured to have met normal distribution and homogeneity of variances. Also, descriptive statistics (frequencies and percentages), including all graphical results were performed on the same statistical platform.
Shannon diversity index (H) was used to measure the diversity of tree species in gaps of different sizes (Harper 1999):
where n is the total number of encountered of individual species, ni is the total number of individuals of taxon i, In = Log base n.
Shannon Evenness was used to measure the ecological distribution of tree species within each gap size community (Hammer et al. 2001):
where e is evenness, H is species diversity, S is the total number of presented species. Simpson index (SI) was used to measure tree species dominance in gaps of different sizes (Harper 1999):
where D is dominance, ni is number of individuals of ith taxon, n is the total number of encountered of individual species.
Berger-Parker index (B-P) was used to measure the numerical importance of the most abundant tree species within gaps of different sizes (Berger and Parker 1970):
where Nmax is the total number of individuals of the most abundant tree species. n is the total number of encountered of individual species. Chao1 diversity index (C-1) was used to measure total richness of tree species in gaps of different sizes (Harper 1999):
where S is the total number of presented species, F1 is the number of singly regenerated tree species in gaps, and F2 is the number of double regenerated tree species in gaps.
Sorensen Similarity Coefficient (SSC) was used to measure tree species similarity of natural regeneration composition between paired gaps of different sizes (Raup and Crick 1979)
where SSC is Sorensen Similarity Coefficient, M is the total number of mutually shared similar tree species of the comparing gaps of different sizes, N is the total number of individual tree species at differing gap sizes in a column with presence in just one row of species frequency.
Results
Effects of logging gaps on the composition of natural regeneration
A total number of 480 individuals belonging to 34 species from 17 families and 30 genera were enumerated in the study (Table 2). Fabaceae and Meliaceae were the most common families with 5 representatives each followed by Malvaceae and Rubiaceae families with 3 species each.
For guild, pioneers topped tree species composition with 15 species, followed by non-pioneer light demanding (NPLD) with 13 species, while shade tolerant attained the lowest count of 6 species. With Conservation Status of tree species, 16, 11, and 7 species were acknowledged as Lower Risk Least Concerned (LRLC), Vulnerable (V) and Lower Risk Near Threatened (LRNT) tree species, respectively, but under Utilisation Status, higher count of 16 species was recognised as Lesser-Used (LU) tree species compared to lower counts of 6 species each for Lesser-Known (LK), Commercial (C), and Premium (P) tree species were observed. Furthermore, 30 tree species belonging to 21 families were evaluated within large size gaps, whereas 20 tree species belonging to 18 families, and 14 tree species belonging to 11 families were recorded within medium size and small size gaps, respectively.
Even though medium size gaps recorded the highest abundance of individuals (173) followed by small size (156) and large size (151) gaps (Table 2). Small size gaps (4370 trees/ha) achieved the highest significant (p < 0.05) mean regeneration density while medium size gaps (2540 trees/ha) enumerated the lowest significant (p < 0.05) density alongside large size gaps (805 trees/ha) recording mean regeneration density which was not significantly different (p > 0.05) from the scores of the former and latter gap sizes (Fig. 3). Meanwhile, Khaya ivorensis was unique to small size gaps, whereas Albizia zygia, Gilbertiodendro limba, and Terminalia ivorensis were only present in medium size gaps. Also, Albizia ferruginea, Aningeria robusta, Bombax buonopozense, Entandrophragma angolense, Funtumia elastica, Heretiera utilis, Khaya anthotheca, Monodora myristica, Nauclea pobeguinii, Terminalia superba, and Tetraprura tetraptera were exclusively present within large size gaps.
In addition, for the three compared groups of gap sizes in species similarity tests, all attained similarity indices showed values higher than 0.5 (Table 3). This indicates that respective comparing paired gap sizes mutually shared over 50% similar tree species in natural regeneration composition. Besides, comparing the estimated SSC indices between paired small size × large size and paired small size × medium size (< 0.65) with paired medium size × large size (> 0.65), the result depicted that the bigger the sizes of the paired gaps, the higher the SSC value.
The abundances of individual tree species within each gap size are presented by their frequencies and relative densities, including the absence of tree species indicated by Not Available (N/A) and zero (0.00), respectively. Columns show tree species names with their authors, trade codes and assigned taxonomic families. Guilds (functional groups based on shade tolerance mechanisms) of tree species comprising Pioneers—light-tolerant (gap or light requiring germinates), NPLD—non-pioneer light demanding species (intermediary light/shade requiring germinates dependent on growth stage) and Shade – shade-tolerant (shade or low light requiring germinates) tree species (Hammond et al. 2021), including other ecological classifications based on (1) Conservation Status (CS) comprising V—Vulnerable tree species (high risk species of possible extinction in the medium-term future as their presence are threatened but not endangered, hence require stricter exploitation controls to avoid over-exploitation), LRNT—Lower Risk Near Threatened tree species (less threatened species that are likely to be vulnerable soon, hence require some form of exploitation controls to avoid overharvesting) and LRLC—Lower Risk Least Concerned tree species (species of less concern for overexploitation or threat, hence require full compliance of the sustainable harvesting practice), and (2) Utilisation Status (US) comprising P—Premium tree species (species of superior quality value), C—Commercial tree species (species of good quality value), LU—Lesser-Used tree species (species of satisfactory value) and LK—Lesser-Known tree species (lower-risk species that are yet to be exploited) according to Oteng-Amoako (2006) detailed descriptions of 100 Tropical African Timber Trees from Ghana.
Gray highlighted tree species were present across all studied gap sizes. Under Oteng-Amoako (2006) tree species classification systems, the Conservation Status classification adopted globally accepted species categorization principles of The Convention on International Trade in Endangered Species (CITES) (2003) and The International Union for Conservation of Nature (IUCN) Red List of Threatened Species (2004) in relation to species growing stock, volume extracted and utilisation status in Ghana; in contrast, the Utilization Status classification was based on 7 evaluation criteria: 1. Period of export (Long = 50 + yr., Short = < 50 yr., Recent = < 20 yr., Local); 2. Quality (Very high, High, Acceptable, Local); 3. Value of export price (Very high, High, Acceptable, Local); 4. Demand for export i.e., Volume (Frequent, Regular, Irregular, Occasional, Local); 5. Forest availability i.e., Growing Stock (Abundant, Frequent, Sparse, Rare); 6. Exploitation i.e., Production Volume (Very High, High, Moderate, Low, Insignificant) and, 7. IUCN Conservation Status (Critically Endangered, Endangered, Vulnerable, Lower Risk Near Threatened, Lower Risk Least Concern) for the respective categorizations. Therefore, Premium (1. Long; 2. High to Very High; 3. High to Very High; 4. Regular to Frequent; 5. Rare to Abundant 6; Low to Very High; 7. Vulnerable to Endangered); Commercial (1. Short; 2. Acceptable to High; 3. Acceptable to Very High; 4. Regular to Frequent; 5. Frequent to Abundant; 6. Moderate to Very High; 7. Lower Risk Near Threatened to Vulnerable); Lesser-Used (1. Recent; 2. Acceptable to High; 3. Acceptable to High; 4. Occasional to Regular; 5. Sparse to Frequent; 6. Low to High; 7. Lower Risk Least Concern to Vulnerable) and Lesser-known (1. Local; 2. Usually acceptable or Local; 3. Local; 4. Local; 5. Rare to Frequent; 6. Insignificant to Low; 7. Lower Risk Least Concern to Lower Risk Near Threatened) tree species. However, tree species bearing (**) were not part of the 100 assessed species by Oteng-Amoako (2006), so the various evaluation of the respective Conservation Status and Utilization Status for these tree species were based on the same principles in Oteng-Amoako (2006) and forest management guidelines at SR.
Effects of logging gaps on the diversity of natural regeneration
From the five biodiversity indices used to evaluate tree species diversity of natural regeneration within three classified gap sizes presented in Table 4, Shannon index (H) proved significant differences among gap sizes at p = 0.001, Simpson's index (1-D) and Berger-Parker index proved significant differences among gap sizes at p = 0.01, whereas Shannon Evenness (e^H/S) and Chao-1 index proved significant differences among gap sizes at p = 0.05. Also, measured species diversity in large size gaps was higher. Small size and medium size gaps significantly shared comparable species diversity across all estimated indices.
Effects of logging gaps on abundance and regeneration density of tree species with contrasting shade tolerance mechanisms
From results in Fig. 4, higher percentage proportions of pioneers (52–76%) compared to NPLD (10–20%) and shade-tolerant (14–31%) were observed across all studied gap sizes. Given 3:1:2, 8:1:1 and 3:1:1 ratio proportion between pioneers: NPLD: shade tree species within the small size, medium size, and large size gaps, respectively. Clearly, pioneers were the most abundant tree species guild across all three studied gap sizes (Fig. 4). Furthermore, in Fig. 5, significant (p < 0.05) differences between pioneers and NPLD tree species for mean regeneration density in medium size and large size gaps were observed. Generally, estimated mean regeneration densities for NPLD were significantly (p < 0.05) lower but not significantly (p > 0.05) different from shade-tolerant in mentioned gap sizes. Meanwhile, there was no significant (p > 0.05) difference among diverse tree species with contrasting shade tolerance mechanisms in small size gaps.
Three functional groups of tree species according to their shade tolerance mechanisms; Pioneer, NPLD—non-pioneer light demanding, and Shade—shade-tolerant tree species in small size, medium size and large size gaps. Bars are showing the respective percentage proportions of functional groups within a specific studied gap size
Multiple comparative analysis of mean regeneration densities of pioneer, non-pioneer light demanding (NPLD) and shade-tolerant (Shade) natural regeneration tree species in three different gap sizes. Overlapping bars are not statistically different at p < 0.05 significance level. Whiskers denote means (n = 4) while bars denote standard deviation of means
Effects of logging gaps on the growth dynamics of natural regeneration
Results of analysis of variance test showed significant differences (p < 0.05) among the five classified height groups of natural regeneration tree species in all studied gap sizes (Table 5). Also, guild and guild × height group interaction became the significant variables that imposed variations in natural regeneration tree species in small size gaps at p < 0.05 significance level. Contrarily, these sources of variation could not drive any variation among natural regeneration tree species in medium size and large size gaps, respectively at p < 0.05 significance level (Table 5).
Furthermore, it could be observed that height class V (151–200 cm) in small size gaps was grossly absent across all guilds of tree species (Table 6). By contrast, in large size gaps, every considered height class (i.e., I = 0–20 cm, II = 21–50 cm, III = 51–100 cm, IV = 101–150 cm, and V = 151–200 cm) was fully represented across all guilds (Table 6). Similarly, regeneration of pioneer and shade-tolerant tree species in medium size gaps, fully attained all evaluated height growth classes; however, regeneration of NPLD tree species encountered inconsistent height growth, particularly evident in height classes II and V that were found completely missing in medium size gaps. In the study, shade-tolerant tree species recorded the lowest mean height of 12 ± 7.78 cm (mean ± SD) in large size and, surprisingly, achieved the highest mean height growth record of 187 ± 18.57 cm in medium size gaps. Meanwhile, all guilds attained the same means for height class I within small size gaps, respectively. Pioneer tree species obtained the lowest mean height of 15 ± 4.54 cm in medium size gaps and the highest comparable mean height of 178 cm in both medium size (± 16.88) and large size (± 15.56) gaps. Also, NPLD tree species recorded 15 ± 5.51 cm and 161 ± 10.92 cm as the lowest and highest mean height growths, respectively, in large size gaps. Moreover, only one regeneration was recorded for pioneer and NPLD tree species, respectively, at height class IV (101–150 cm) in small size gaps (Table 6).
Effects of logging gaps on natural regeneration with different conservation and utilisation statuses
For Conservation Status (Fig. 6), LRLC tree species were the predominant species in all three gap sizes with the highest occurrences in medium size gaps (73%), while the occurrences of LRNT and V tree species were comparatively lower across gaps ranging between 11 and 29% of the overall regeneration composition. Additionally, except for the regeneration abundance of LU tree species that was independent of the gap size factor, abundances of the other commercial tree species based on Utilisation Status (Fig. 6) depended strongly on gap size. LK tree species attained the highest abundance (52%) in medium size gaps, while P and C, as valuable tree species, jointly recorded dominant regeneration (37%) within small size gaps, but then again, they were individually higher than the abundance of LK tree species. It is obvious that small size gaps facilitated successful natural regeneration and maintained a well-balanced proportion among LRLC/LRNT/V tree species (47%/29%/24%) and also for LU/P/C/LK (47%/19%/17%/16%) as clearly shown in Fig. 6. This result presents helpful guidance for forest managers on the relationship between gap size and different suites of tree species undergoing natural regeneration after logging operations.
Different suites of tree species in Ghana according to their Conservation Status (left): Lower Risk Least Concerned (LRLC), Lower Risk Near Threatened (LRNT) and Vulnerable (V) tree species representatives, and Utilisation Status (right): Premium (P), Commercial (C), Lesser-Used (LU) and Lesser-Known (LK) tree species within gaps of different sizes (small size, medium size and large size)
Discussion
This study explicitly shows the potent impacts of different sizes of logging gaps on species composition, maintenance of species diversity, and growth dynamics of naturally regenerating tree species. Also, the study describes the integral roles these species play in facilitating the economic and ecological agenda of sustainable forest management after logging disturbances in a tropical forest.
Evaluation of gap sizes following different logging regimes
There were significant differences among studied gap sizes (Table 1). In the presented design of gap analysis, large size gaps with the average size of 575 ± 186.79 m2 (i.e., doubled the size of investigated medium size gaps) were noticed to be statistically different from medium size gaps (with the size of range 201–300 m2, on average 227 ± 23.11 m2, again doubled the size of investigated small size gaps) followed by small size gaps (25–200 m2, average size of 119 ± 47.89 m2). The variation found between large size gaps and medium-small size gaps was due to expansive canopy openings. This finding was a result of the high number of felled trees leading to the creations of relatively wider openings within forest canopies noticed at the areas where large size gaps were identified. This finding agrees with De Carvalho et al. (2017) that logging disturbances differ in spatial scale or intensity, ranging from small-scale to heavy-scale operations. Similarly, in temperate European forests, big gaps were formed from group selection system, while small and medium gaps were formed from singleton and doubleton treefalls (Hammond and Pokorný 2020b). For instance, under typical logging regimes in SR, it was detected that legal TUC holders, on average, caused multiple treefalls (i.e., averagely between 600 and 700 allowable cut trees per 128 ha compartment), resulting in the creations of large size gaps while illegal operators typically caused discriminatory single treefalls of Minimum Felling Diameter (MFD) far below the instituted 50 to 110 cm range (Adam et al. 2006; Oduro et al. 2011) for different types of tree species within the reserve. These logging regimes of illegal operators usually bring about the creations of small size to medium size gaps simultaneously depending on the type of species felled. Our observation is consistent with other studies conducted in Nkrabia (Herrmann 2011) and Bia Tano (Hammond et al. 2021) forest reserves of Ghana and elsewhere in Europe (Hammond and Pokorný 2020b). More so, Martins and Rodrigues (2005) have also argued that 'gap size" characteristic depends vastly on the magnitudes of logging disturbances, in those open spaces within forest canopies and gap size correlates positively with canopy openness. Contrarily, De Carvalho et al. (2017) suggested that logging only causes slight changes in canopy openness. Again, features of stand vegetation composition like canopy architecture and geometry were also reported to have a strong relationship with the mean canopy gap size (Hawthorne et al. 1995). Therefore, this knowledge could be a useful element for comparing the ecological impact of different logging approaches, especially from a tropical viewpoint.
Effects of gap sizes on the composition and diversity of natural regeneration
The total number of tree species recorded in medium size gaps was higher than those enumerated in small size and large size gaps. On the other hand, the comparatively higher difference between the number of diversified tree species encountered in large size (over 25 species) and medium size (20 species) gaps was due to the continuous ecological provision of a broad spectrum of optimal light conditions within medium size to large size gaps that offered the specific required light amounts for growth of diverse participatory tree species in such relatively big gaps. In a related account, Cordonnier et al. (2018) mentioned that creating larger mean gap sizes encourage early successional species and promote species coexistence because such gap sizes mechanistically invoke species coexistence mechanisms through their routine higher light interception and light-use efficiency. However, the lower presence of different tree species in small size gaps (14 species: less than half of the overall encountered tree species) resulted from keen competition among seedlings for less available light (Sapkota and Oden 2009) for germination, establishment, growth and development. Our result strongly opposes the findings of Bobiec (2007) that gap size cannot significantly influence the composition of gap regeneration under mixed-species forests. Even though we anticipated a higher number of individuals within large size gaps due to the presence of a fairly higher level of microclimatic light condition: an essential resource for plant growth, rather our results support previous findings that large size gaps (> 600 m2) only promote tree species regeneration during the early growth stage (i.e., germination and establishment) (Lu et al. 2015) and are seemingly not suitable for overall seedling growth and regeneration abundance of different tree species due to weak light competition among species (Sapkota and Oden 2009). Subsequently, an undesirable effect of large size gaps on seedling growth had been reported in the Czech Republic (Hammond et al. 2020) and Indonesia (Tuomela et al. 1996). This finding corroborates an earlier study of Agyeman et al. (2010), who recorded poor regeneration of different suites of tree species under the light condition above 70% irradiance within large gaps. The felling schedule (timing of logging operation) coupled with the low intensity of logging disturbance favoured small size gaps to obtain the highest mean regeneration density in this study.
To Akoto et al. (2015) interpretations of SSC values: (1) if the SSC index is less than 0.5, then compared pair share different species but (2) if the SSC index is greater than 0.5, then compared pair share similar species. Therefore, from this understanding, our species similarities test results revealed that all evaluated gap sizes (0.59–0.68) shared similar species in natural regeneration composition. This observation corroborates the findings of Schnitzer and Carson (2001) that in many tropical forests, gaps play a similar role in maintaining tree species diversity. The estimated SSC index for compared small size × large size was lower than the obtained SSC index (0.78) for the same paired gap sizes in another study (Hammond et al. 2020). Therefore, our results do support the assumption that species diversity in gaps is only temporary and that flora species diversity immediately increase rapidly soon after gap creation (Whitmore 1989; Agyeman et al. 1999), because ecological conditions of newly created gap always resemble that of the natural forest settings and gradually with time, gaps of different sizes or shapes would have similar richness in plant species (Muscolo et al. 2014). Contrary to our findings, De Carvalho et al. (2017) observed that different magnitudes of logging disturbance triggered variations in species composition of tree regeneration in gaps, while Whitmore (1989) and McCarthy (2011) assert that gap size is the major determining factor in post-disturbance tree species composition. It is of the fact that gap size alone cannot determine the composition of natural regeneration of tree species within gaps following logging disturbances but plays a key role in the organisation of different species in gaps. Differential shade tolerance mechanisms of participatory tree species also play decisive roles in species survival and growth mechanisms. This to a more considerable extent, poses a strong influence on species composition in the post-disturbance tree regeneration process. For example, Nagel et al. (2010) discovered that the coexistence of Fagus sylvatica and Abies alba in the Dinaric Mountains of Bosnia–Herzegovina depended substantially on their individual's life history attributes, rather than the gap-size variable of their thriving gaps. Also, sharing similar forest vegetation could have attributed substantively to the comparable species composition among small size, medium size, and large size gaps.
Measured higher species diversity in large size gaps than in other studied gap sizes is not different from the results of other studies (Pourbabaei et al. 2013; Hammond et al. 2020). However, this result disagrees with Agyemang et al. (1999), who stated that small felling gaps enhance floristic diversity, but large felling gaps reduce biodiversity by simplifying forest ecosystems after tree exploitation. The species diversity results in this study generally project gaps as an optimum silviculture technique for stimulating biodiversity in the forest and, at the same time, demonstrate the positive effects of logging disturbances on tree species diversity. But most importantly, presenting large size gaps as preferable gap size for enhancing species diversity. Many studies have also documented this assertion (e.g., Pourbabaei et al. 2013; Muscolo et al. 2014; Hammond et al. 2020). In short, for gap dynamics and its associated regeneration, gap size is a valuable parameter in predicting species distribution patterns, diversity, and richness within gaps under any forest ecosystem in the tropics.
Creation of skid trails, uncleared offcuts, presence of soil surface disturbances, and unintentional felling of other tree species from casualties during logging operations in the study area explain the overall lower species richness (34 species) encountered in this study compared to the relative higher species richness (63 species) of another forest area in Ghana, where diversity of tree species in gap regeneration following different logging disturbances was studied (Hammond and Pokorný 2020a). This finding brings to light the improper management of logging operations at SR, which has led to a lower bioquality state of the forest and the degradation of the natural state of floral biodiversity. This observation substantiates a claim by Hawthorne and Abu-Juam (1995) that logging practices in Ghana are inappropriately managed. In the long run, there would be the possibility of well-dispersed logging disturbances, which would perhaps deteriorate the current state of tree species composition and diversity. Thus, the resilience of forest regeneration and maintenance of a more vigorous forest mosaic is likely to be challenged soon. In the same way, Vaglio et al. (2016) also expressed that the impacts of both selection and illegal logging practices often hamper tree species protection status in tropical forest reserves. They further affirmatively declared the West African forests, including Ghana, as critically endangered forests for biodiversity conservation (Vaglio et al. 2016).
Meanwhile, there was a detection of uncleared offcuts, moderate presence of plant residuals, snags, and skid trails within growing spaces of most studied gaps, prominently noticed at compartments 18 and 19. These conditions presented adverse growing conditions like soil compaction and growth barriers at regeneration sites. This finding probably could also be related to the occurrences of low species diversity across all studied gaps. Therefore, this study confirms an earlier submission that most logging sites in Ghana appear untidy and scattered after logging operations and, loading areas experience soil erosion and poor tree regeneration (Hawthorne and Abu-Juam 1995). In effect, certain species do not regenerate following such soil disturbances Duah-Gyamfi et al. 2014). Likewise, Swaine and Hall (1983) also associated soil disturbance conditions as the underlying factors for variations of species diversity in gaps. In an overall contrast to our results, Ashton (1978) claimed that species diversity is universally greater under mature stands with uneven canopy structure than under full canopy gaps in tropical forests.
Effects of gap sizes on distribution and patterns of guild and height growth of natural regeneration
The high total abundance (above 52%) (Fig. 4) and higher representations (Table 2) of pioneers across all three studied gap sizes, illustrate the competitive advantage of this guild of tree species in forests undergoing gap regeneration following various degrees of logging disturbances. Also, result demonstrates that pioneers generally grow faster in gaps but in young logging gaps of below five years old under tropical forests, they exhibit greater growth abundance compared to NPLD and shade-tolerant tree species. Likewise, Duah-Gyamfi et al. (2014) also reported an outstanding growth abundance of pioneer tree species in 0.5, 10 and 33-months old logging gaps. The high levels of light ensuing from logging operations favoured regeneration of pioneer tree species at understoreys compared to other guilds of tree species (Whitmore 1989; Duah-Gyamfi et al. 2014). Besides, moderate soil disturbances within gaps triggered the high proliferation character of pioneer species (Darrigo et al. 2016). This finding has been reported in western Brazil, where heavily disturbed soil habitats like log landings and secondary roads under wide gap openings promoted higher density of commercial pioneer timber species like Cedrela odorata, Jacaranda copaia and Handroanthus serratifolius even after eight years of selective logging (De Carvalho et al. 2017). Therefore, this result proves that gaps are favorites growing sites for light-adapted tree species. Comparably, other papers have also mentioned this (Lu et al. 2015; Darrigo et al. 2016). In converse, our results were entirely opposite to the results of Herrmann (2011) and Hammond and Pokorný (2020a), who accounted lower percentage composition of pioneers but higher percentage composition of shade-tolerant tree species in natural regeneration at various growth stages within gaps of different sizes. Shade-tolerant tree species were found to be higher in small size gaps. Martins and Rodrigues (2005) equally found an extreme concentration of late secondary species (shade-tolerant species) in small size gaps compared to any other studied gap sizes. Moreover, the biological swift light response character of pioneers coupling with their aggressive gap colonisation potential could be the reason why regeneration densities of pioneers were significantly higher than ‘intermediate light-demanding’ NPLD tree species in medium to large size gaps in this study. In mixed central European forests, a similar observation was encountered (Hammond et al. 2020). In addition, no significant difference was recorded between pioneers and shade-tolerant tree species in medium size and large size gaps because there was a good balance between the rate of pioneer mortality and the rate of shade-tolerant recruitment and establishment. In another tropical forest, shade-tolerant tree species were pronounced as opportunistic fast replacers of gap-dependent pioneers in forest gap environments (Hawthorne et al. 2012; Hammond et al. 2021).
Again, in this study, gap size was observed to be a significant feature that caused variations in height growth among naturally regenerated tree species in gaps (Table 5). By contrast, Sapkota and Oden (2009) observed no significant difference in height growth among various regenerating tree species within large size gaps. Our result exemplifies an interesting positive relationship between seedling height and light environments (gaps) and suggests that light is crucial to height growth. This finding validates a notion that height growth has a prompt response to light availability following canopy opening (Collet and Chenost 2006). Aside from this, the absence of height growth V in small size gaps and the assessment of all classified height growth groupings in large size gaps (Table 6) clearly portrays the limiting light factor in small size gaps, which could not support tree species in natural regeneration to reach 151 cm and over height. However, in large size gaps, the possibility of regular supply of ecologically required light factor for various suites of naturally regenerated tree species was the reason behind the full attainment of all considered height growth groupings among encountered tree species. The distribution pattern and dynamics of different height growths of natural regeneration of tree species in logging gaps of different sizes in this study attest that gap size is indeed a significant light resource regulator (Agyeman et al. 1999; Hammond and Pokorný 2020c). Briefly, this result illustrates that light resource has significant influences on height growth of tree regeneration (Dobrovolný and Cháb 2013).
Effects of gap sizes on conservation and utilisation statuses
The less successful regeneration of V tree species across all gap sizes compared to the excellent regeneration of LRNT and LRLC tree species validates a statement made by Hawthorne and Abu-Juam (1995) that uncontrollable timber exploitation has destroyed some yielding areas of most forest reserves in Ghana, rendering about 50% of these areas as “mostly degraded” status. For the high recent deforestation rate in most tropical forests (Asamoah et al. 2020), we suggest ecologists and foresters separate forest restoration silviculture systems following logging disturbances from the forest protection silviculture system. This is because in situations whereby forest management objectives focus on enhancing and sustaining tree biodiversity, achieving these objectives jointly in the same forest ecosystem often poses challenges. Besides, the autecology of timber tree species responses to disturbances is often complicated (Karsten 2014; De Carvalho et al. 2017) because of species-specific interactions and adaptations to the factors (both living and non-living) of its environment for distribution and abundance, alongside the ecological relationship between species-specific requirements and environmental tolerances of individuals to the geographic distribution of the species (Walter and Hengeveld 2014). For this reason, Foggie (1962) categorically stated that in a quest to achieve sustainable forest management (i.e., holistic economic, ecological and social objectives), particularly when considering conservation priority from the quiet side, hard to accomplish this goal successfully when pursuing exploitation (i.e., economic) and protection (i.e., ecological) agenda at the same time. Nonetheless, Hawthorne and Abu-Juam (1995) pointed out that combining the protection of rare tree species and sustainable biodiversity had never been a stated objective in tropical forest management for years in the protection of forest reserves until quite recently when protection of the whole forest ecosystem has become a necessity for the conservation of commercial tree species. In the light of this, Hawthorne and Gyakari (2006) recommended the use of Conservation Star Ratings (CSR) (i.e., is a robust scientifically accepted timber species conservation scheme for evaluating forest trees in Ghana ranging from the rarest to common tree species) for assessing the conservation of commercial tree species in Ghana (Hammond and Pokorný 2020a). This locally devised conservation system helps to monitor and evaluate various commercial timber species' conservation status, curb forest degradation menace, and control the creaming of commercial timber species. Most importantly, CSR serves as a helpful guide for assessing the overall conservation of the biodiversity legacy of forest areas. Furthermore, it was observed that V─Entandrophragma angolense, Khaya spp. and Terminalia ivorensis which are also classified as Scarlet Stars (species with high conservation priority) including LRNT─Aningeria robusta, Canarium schweinfurthii and Pycnanthus angolensis which are also Red Stars (species with some conservation priority) are still being logged legally with special logging permit from the Forestry Commission of Ghana and illegally by some unscrupulous persons in SR.
Additionally, the lower regeneration records of C and P tree species in all three groups of surveyed gap sizes could be linked to either absence or presence of inadequate residual parent tree sources due to overexploitation of adult (mother) trees. In the same account, Darrigo et al. (2016) stated that the distribution of early seedlings recruitment is related to the presence of a parent plant. Oteng-Amoako (2006) outlined P − Khaya spp. and Terminalia ivorensis species as overly exploited due to their very high demands in local and international markets. Likewise, C − Aningeria robusta, Nauclea diderrichii and Terminalia superba are having the same harvesting issue, while LU − Albizia ferruginea species is less extracted because of its low local patronage and low international market demands. Although in Ghana, forest management is exclusively based on a National Forest Management guideline called polycyclic silviculture system aimed to foster less damage to residual forest trees and ensure sufficient tree regeneration after logging. This silviculture scheme involves a selection system of tree harvesting subjective to species-specific 50–110 cm MFD limit and the application of a selected number of stems as allowable cut under 40-year felling cycle (Adam et al. 2006). Yet, the current practice is such that this selection system often removes only the most highly valued tree species and does not provide any appropriate restoration strategies for successful regeneration of harvested tree species (Oduro et al. 2011). According to Taylor (1960), the application of MFD limit in logging operations helps to prevent and protect immature trees from logging. Unfortunately, in SR some law-breaking loggers sometimes harvest high valued immature commercial tree species.
The truth is that on paper, logging regulations look so promising, but their implementation in the forest is low and often challenging due to the failure of the forest authorities to ensure an effective control system adequately and manage the forest sustainably. Likewise, in earlier studies (e.g., Bach et al. 1995), authors discovered that, in Ghana, though documentation of strict regulations on forest planning and management for effective logging operations within forest reserves exist for TUC holders to operate on a 40-year felling cycle, the amount and location of timber that could be logged is the sole responsibility of the Forest Services Division. The division has the statutory right to issue Allocation Quota Permits (AQP) for the execution of logging operations. Unfortunately, in reality, this AQP is a complete mirage. The reasons being that, in practice, (1) about one-third or even lower of the total amount of trees above 50 cm are normally allocated for logging and (2) timber companies often unreservedly remove only the most valuable species based on market demands which reveals a clear violation of the issuance of AQP. In measures to clamp this menace down, Bach (1999) and Bach et al. (1995) have recommended that forest authorities should strictly ensure that logging operators conform to the stepwise procedures and principles outlined in the logging manual. Also, activities of logging operators should be monitored regularly during and after logging operations by well-equipped forest officers at logging sites. In addition, the administrative machinery should be strengthened to strictly uphold the statutory forest management laws without any fear or favour. Notwithstanding, a more robust and regular monitoring routine should be reinforced.
Furthermore, C − Antiaris toxicaria and P − Milicia excelsa species performed comparatively better in small size gaps than in any other gap sizes. This finding substantiates Hawthorne (1993), who detected enhanced regeneration within small size gaps at Bia-South Forest Reserve after three years of timber exploitation. However, LU and LK tree species maintained an appreciable higher regeneration across all gap sizes: their potential to regenerate quickly after logging disturbances was species-specific. This opinion contradicts the findings of another study that logging disturbances reduce advance regeneration in canopy gaps (Swaine and Agyeman 2008).
Conclusions
Logging gaps of different sizes are important growing niches for rich composition, sustainable succession and conservation of natural regeneration, and promotional grounds for valuable growth dynamics among naturally regenerating tree species with different shade tolerance mechanisms in tropical forests.
Mature stand composition and the AQP for timber harvesting influenced the scale of operations, mechanisms, intensity, and frequency of logging, while biological tree form and number of treefalls determined mean gap sizes. Gap size significantly explained variations of species diversity, richness and height growth dynamics of natural regeneration in gaps. Species diversity was significantly higher in large size gaps followed by medium size and small size gaps. Positively, logging influenced the natural regeneration of different suites of timber trees in gaps of different sizes.
For guilds: pioneer tree species preferred medium to large size gaps while shade-tolerant tree species preferred small size gaps for their regeneration abundance. The effects of logging on canopy opening, soil disturbances and stand vegetation disturbances greatly enhanced natural regeneration, preferably pioneer tree species. V and LRNT tree species under Conservation Status and, P and C tree species under Utilisation Status preferred small size gaps for their proliferation and conservation. Nonetheless, regeneration of NPLD, LRLC and LU tree species were independent of gap size. In brief, it was observed that natural regeneration of tree species in logging gaps of different sizes depended greatly on their ecology (e.g., light demanding vs. shade-tolerant) rather than their conservation status or economic interest. Also, reduced populations of higher valued commercial species, to name a few; Aningeria robusta, Khaya spp., Nauclea diderrichii, Terminalia spp. and Turraeanthus africanus species may result in an increasing shift to harvest lesser valued species. This is symptomatic of overexploitation of potential mother trees and unless checked, it will result in a collapse the commercial forestry sector. In addition, small size gaps guaranteed a more stable microsite for both excellent regeneration performances and balanced proportions of different suites of natural regeneration tree species. Yet, medium size gaps became the most suitable and optimal regeneration niches for abundant seedlings' growth.
Therefore, we recommend the single tree-based selective logging to ensure the creations of small to medium size gaps (200–300 m2) through adjustments to the logging permit process, revision of AQP, and strict adherence to the 40-year polycyclic selection system, along with more dedicated and effective enforcement and monitoring. Changes along these protocols would tremendously facilitate natural regeneration of different suites of timber species and the early stimulation of seedling growth, resulting in the improvement of overall biodiversity conservation associated with the forest, more sustainable forest harvests and more income to those who receive logging permits. Hence, this study will serve as a guideline for future forest management on post-logging effects on natural regeneration in gaps.
References
Abu-Juam M, Hawthorne W (1994) Evaluation of the 1993 botanical survey of Area 'E' and other parts of Subri FR. Accra: Department of Forestry, p 10
Adam KA, Pinard MA, Swaine MD (2006) Nine decades of regulating timber harvest from forest reserves and the status of residual forests in Ghana. Int for Rev 8(3):280–296
Agyeman VK, Swaine MD, Thompson J (1999) A comparison of gap microclimates in two forest types in Ghana. Ghana J for 7:51–69
Agyeman VK, Swaine MD, Thompson J, Kyereh B, Duah-Gyamfi A, Foli EG, Adu-Bredu S (2010) A comparison of tree seedling growth in artificial gaps of different sizes in two contrasting forest types. Ghana J for 26:14–40
Akoto SD, Asare A, Gyabaa G (2015) Natural regeneration diversity and composition of native tree species under monoculture, mixed-culture plantation and natural forest. Int Res J Nat Sci 3(2):24–38
Appiah M (2013) Tree population inventory, diversity and degradation analysis of a tropical dry deciduous forest in Afram Plains, Ghana. For Ecol Manag 295:145–154
Asamoah O, Kuittinen S, Abrefa-Danquah J, Quartey ET, Bamwesigye D, Boateng MC, Pappinen A (2020) Assessing wood waste by timber industry as a contributing factor to deforestation in Ghana. Forests 11:939
Ashton PS (1978) Crown characteristics of tropical trees. In: Tomlinson PB, Zimmermann MH (eds) Tropical trees as living systems. Cambridge University Press, Cambridge, pp 591–616
Bach CF (1999) Economic incentives for sustainable management: a small optimal control model for tropical forestry. Ecol Econ 30(2):251–265
Bach CF, Gram S, Helles F, Kanafani N, Treue T (1995) Incentives for sustainable production of tropical timber. Copenfhagen: The Royal Veterinary and Agricultural University, Department of Economics and Natural Resources, pp 3–13
Berger WH, Parker FL (1970) Diversity of planktonic foraminifera in deep-sea sediments. Scince 168:1345–1347
Bobiec A (2007) The influence of gaps on tree regeneration: a case study of the mixed lime-hornbeam (Tilio-Carpinetum Tracz. 1962) communities in the Białowieża Primeval Forest. Polish J Ecol 55:441–455
Brammer H (1962) Soils. In: Wills JB (ed) Agriculture and Landuse in Ghana. Oxford University Press, London, pp 88–126
Collet C, Chenost C (2006) Using competition and light estimates to predict diameter and height growth of naturally regenerated beech seedlings growing under changing canopy conditions. Forestry 79(5):489–502
Convention on International Trade in Endangered Species of Wild Fauna nd Flora, CITES (2003). Accessed at https://cites.org/eng/node/5021 on 7th January, 2020
Cordonnier T, Kunstler G, Courbaud B, Morin X (2018) Managing tree species diversity and ecosystem functions through coexistence mechanisms. Annals for Sci 75:65
Darrigo MR, Venticinque EM, dos Santos FAM (2016) Effects of reduced impact logging on the forest regeneration in the central Amazonia. For Ecol Manag 360:52–59
De Carvalho AL, d’Oliveira MVN, Putz FE, de Oliveira LC (2017) Natural regeneration of trees in selectively logged forest in western Amazonia. For Ecol Manag 392:36–44
Diame GLA (2007) Ethnobotany and ecology of plants of importance reproductive health: A case study of Subri River Forest Reserve in the Western Region of Ghana. PhD dissertation. Cape Coast: University of Cape Coast, p 10
Dobrovolný L, Cháb M (2013) Ecology of beech regeneration in the allochthonous spruce stands–a case study. Acta Uni Agric Silvic Mendelianae Brun 61(5):1261–1268
Duah-Gyamfi A, Kyereh B, Adam KA, Agyeman VK, Swaine MD (2014) Natural regeneration dynamics of tree seedlings on skid trails and tree gaps following selective logging in a tropical moist semi-deciduous forest in Ghana. Open J for 4(1):49
Foggie A (1962) Forestry in the agricultural economy. In: Willis BJ (ed) Agriculture and land use in Ghana. Oxford University, Oxford, p 232
Forestry Commission of Ghana, FC (2002) A Management Plan for Subri River Forest Reserve. Takoradi: Wstern Region Forest Services Division, p 10
Fox TJ, Knutson MG, Hines RK (2000) Mapping forest canopy gaps using airphoto interpretation and ground surveys. Wildl Soc Bull 28(4):882–889
Hall JB, Swaine MD (1981) Distribution and ecology of vascular plants in a tropical rain forest. Junk publishers, Hague, p 383
Hammer Ø, Harper DA, Ryan PD (2001) PAST: paleontological statistics software package for education and data analysis. Palaeontol Electronica 4(1):9
Hammond ME, Pokorný R (2020a) Diversity of tree species in gap regeneration under tropical moist semi-deciduous forest: an example from Bia Tano Forest Reserve. Diversity 12(8):301
Hammond ME, Pokorný R (2020b) Effects of gap size on natural regeneration and micro-environmental soil conditions in European beech (Fagus sylvatica L.) and Norway spruce (Picea abies (L.) Karst) dominated mixed forest. Plant Soil Environ 66(12):607–615
Hammond ME, Pokorný R (2020c) Preliminary assessment of effect of disturbance on natural regeneration in gaps of different sizes. J for Sci 66:185–196
Hammond ME, Pokorný R, Dobrovolný L, Hiitola N, Friedl M (2020) Effect of gap size on tree species diversity of natural regeneration–case study from Masaryk Training Forest Enterprise Křtiny. J for Sci 66:407–419
Hammond ME, Pokorný R, Okae-Anti D, Gyedu A, Obeng IO (2021) The composition and diversity of natural regeneration tree species in gaps under different intensities of forest disturbances. J for Res 32(5):1843–1853
Harper DAT (1999) Numerical Palaeobiology: computer-based modelling and analysis of fossils and their distributions. Wiley, New York, p 468
Hawthorne WD (1993) Forest regeneration after logging: Findings of a study in the Bia South Game Production Reserve, Ghana. Overseas Development Administration, London, pp 10–48
Hawthorne WD (1995) Ecological profiles of Ghanaian forest trees. Oxford Forestry Institute, Oxford, p 29
Hawthorne WD, Abu-Juam M (1995) Forest protection in Ghana with particular reference to vegetation and plant species. IUCN Publications Services Unit, Cambridge, p 16
Hawthorne WD, Gyakari N (2006) Photoguide for the forest trees of Ghana: a tree-spotter’s field guide for identifying the largest trees. Oxford Forestry Institute, Oxford, pp 18–348
Hawthorne WD, Jongkind CC (2006) Woody plants of Western African forests, A guide to the forest trees, shrubs and lianes from Senegal to Ghana. Royal Botanic Gardens, Kew, pp 10–23
Hawthorne WD, Sheil D, Agyeman VK, Juam MA, Marshall CAM (2012) Logging scars in Ghanaian high forest: towards improved models for sustainable production. For Ecol Manag 271:27–36
Herrmann TD (2011) Inventory of natural regeneration and the recovery of logging gaps in the Nkrabia forest reserve in Ghana: a comparison between chainsaw milling and conventional logging. Bachelor thesis. Leeuwarden: University of Applied Sciences Van Hall Larenstein, p 13–30
Karsten RJ, Meilb H, Larsen JB (2014) Regeneration and management of lesser-known timber species in the Peruvian Amazon following disturbance by logging. For Ecol Manag 327:76–85
Lu D, Zhu J, Sun Y, Hu L, Zhang G (2015) Gap closure process by lateral extension growth of canopy trees and its effect on woody species regeneration in a temperate secondary forest, Northeast China. Silva Fennica 49(5):13–10
Martins SV, Rodrigues RR (2005) Assessing the role of the canopy gap characteristics in the regeneration of shrub and tree species in a semideciduous mesophytic forest in south-eastern Brazil. In: Burk AR (ed) New research on forest ecosystems. Nova Science Publishers, New York, pp 93–112
McCarthy J (2001) Gap dynamics of forest trees: a review with particular attention to boreal forests. Environ Rev 9(1):1–59
Ministry of Lands and Natural Resources of Ghana, MLNR (2012) The state of the world's forest genetic resources. Accra Ghana: Ministry of Lands and Natural Resources, p 20–21
Ministry of Lands and Natural Resources of Ghana, MLNR (2016) Ghana forestry development master plan (2016–2036). Accra Ghana: Ministry of Lands and Natural Resources, p 30–35
Muscolo A, Bagnato S, Sidari M, Mercurio R (2014) A review of the roles of forest canopy gaps. J for Res 25(4):725–736
Nagel TA, Svoboda M, Rugan T, Diaci J (2010) Gap regeneration and replacement patterns in an old-growth Fagus-Abies forest of Bosnia-Herzegovina. Plant Ecol 208(2):307–318
Oduro KA, Foli EG, Mohre GM, Dumenu WK (2011) General aspects of forestry in Ghana. Sustain Manage Trop Rainfor CELOS Manage Syst 14:242–254
Oteng-Amoako AA (2006) 100 tropical African timber trees from Ghana: tree description and wood identification with notes on distribution, ecology, silviculture, ethonobotany and wood uses. Kumasi: Department of Publishing Studies, Kwame Nkrumah University of Science and Technology, p 37-46
Parren MP, de Graaf NR (1995) The quest for natural forest management in Ghana, Côte d’Ivoire and Liberia. Wageningen, Veenman Drukkers, p 37
Pourbabaei H, Haddadi-Moghaddam H, Begyom-Faghir M, Abedi T (2013) The influence of gap size on plant species diversity and composition in beech (Fagus orientalis) forests, Ramsar, Mazandaran Province, North of Iran. Biodiversitas 14:84–94
Raup DM, Crick RE (1979) Measurement of faunal similarity in paleontology. J Paleontol 53(5):1213–1227
Sapkota IP, Oden PC (2009) Gap characteristics and their effects on regeneration, dominance and early growth of woody species. J Plant Ecol 2(1):21–29
Schnitzer SA, Carson WP (2001) Treefall gaps and the maintenance of species diversity in a tropical forest. Ecology 82:913–919
Swaine MD, Agyeman VK (2008) Enhanced tree recruitment following logging in two forest reserves in Ghana. Biotropica 40(3):370–374
Swaine MD, Hall JB (1983) Early succession on cleared forest land in Ghana. J Ecol 71:601–627
Taylor CJ (1960) Synecology and silviculture in Ghana. Thomas Nelson and Sons Ltd, London and Edinburgh, p 418
The International Union for Conservation of Nature IUCN (2004) A global species assessment. In: Baillie JEM, Hilton-Taylor C, Stuart SN (eds) IUCN red list of threatened species. IUCN Publications Services Unit, Cambridge, pp 27–64
Tuomela K, Kuusipalo J, Vesa L, Nuryanto K, Sagala APS, Ådjers G (1996) Growth of dipterocarp seedlings in artificial gaps: an experiment in a logged-over rainforest in South Kalimantan, Indonesia. For Ecol Manag 81(1–3):95–100
Vaglio LG, Hawthorne WD, Chiti T, Di Paola A, Cazzolla GR, Marconi S, Valentini R (2016) Does degradation from selective logging and illegal activities differently impact forest resources? A case study in Ghana. iForest-Biogeosci for 9:354–362
Walter GH, Hengeveld R (2014) Autecology: organisms, interactions and environmental dynamics. CRC Press, New York, pp 1–8
Whitmore TC (1989) Canopy gaps and the two major groups of forest trees. Ecology 70:536–538
Wilks I (1985) The Mossi and the Akans. In: Ajayi JFA, Crowder M (eds) History of West Africa. Longman, Essex, pp 465–502
Yamamoto SI (2000) Forest gap dynamics and tree regeneration. J for Res 5(4):223–229
Acknowledgements
We would like to express our profound gratitude to several individuals and institutions whose contributions have made this study possible. Firstly, to Takoradi Forest Services Division for granting us a forest entry permit and logistics support. Also, Mr Ekow Bentum, Mr Foster, Forest Guards, and Staff of Ghana Primewood Products Ltd. at Subri River Forest Reserve for their unmatched field assistance and release of other relevant information. Mr. William Amoako Debrah, the Western Regional Forest Services Division's cartographer for unleashing territorial information about reserve and forest map production. Also, we are most grateful to Prof. Daniel Okae-Anti of the Department of Soil Science at the University of Cape Coast- Ghana for his immeasurable assistance. Finally, we are very thankful to reviewers for their helpful comments that have improved the overall readability of this paper.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Project Funding: this research was funded by the Internal Grant Agency of Mendel University in Brno (LDF_VP_2019015) and the Framework of Bilateral Mobility Program for Traineeship of Doctoral Students, MENDELU.
The online version is available at http://www.springerlink.com.
Corresponding editor: Zhu Hong.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hammond, M.E., Pokorný, R., Abugre, S. et al. Natural regeneration in logging gaps of different sizes in Subri River Forest Reserve (Ghana). J. For. Res. 33, 1157–1174 (2022). https://doi.org/10.1007/s11676-021-01435-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11676-021-01435-4