Abstract
Wilt disease with unknown etiology causes mass mortality in commercial Acacia mangium nurseries in South Sumatra. This pathogen induces symptoms of chlorosis in the lower leaves and develops into the shoots; subsequently, the plants wither and die. This research identifies the pathogenic species causing this wilt disease and to assess its pathogenicity or virulence. Fifteen isolates of Fusarium oxysporum with varying colony sizes and color pigments were recovered from symptomatic A. mangium seedlings. The pathogenicity test showed that all isolates could infect plants with wilt severity reaching 80%, and the pathogen was verified as causing vascular disease. Koch’s postulate was verified by re-isolating the F. oxysporum isolates. The pathogen was confirmed by observing the morphological characters and elongation factor 1-α (tef1-α) gene sequences as F. oxysporum.
Similar content being viewed by others
Introduction
Black wattle (Acacia mangium Willd.) is a flowering tree species native to Papua, West Irian Jaya and Maluku in Indonesia, Papua New Guinea and northeast Queensland in Australia. Under favorable conditions, this species can grow up to 30 m with a diameter of 50 cm. It is cultivated on industrial plantations by large companies for its high levels of quality pulp and a good paper yield (Hedge et al. 2013). In 2018, the total land area controlled by industrial plantation forest companies in Indonesia was 8.67 million hectares, with 81.3% used for plant cultivation, including A. mangium. In addition, in 2017, this species produced the most logs of any other species, reaching 77.5% from a log total of 40,628.8 m3 (Statistics Indonesia 2018).
A major problem in its cultivation is an unknown cause of seedling wilt disease. It occurs during the initial stages of growth and if ignored, can spread widely in a plantation.
Fusarium oxysporum Snyder and Hansen is a soil-borne pathogen with a wide range of hosts and is common in various regions of the world (Bayona et al. 2011; Orr and Nelson 2018; Edel-Hermann and Lecomte 2019), including forests and industrial plantations (Widyastuti et al. 2013). This pathogen attacks all phases of growth (vegetative and regenerative) and survives on plant debris for long periods (Postic et al. 2012; Meena and Roy 2020). It causes vascular wilt or root rot disease in plants (Bayona et al. 2011; Gordon 2017). The symptoms involve chlorosis of the leaves, stunted growth, discoloration of the plant's vascular vessels, and withering, resulting in death (Velarde-Félix et al. 2018; Sun et al. 2019). This species is a dangerous pathogen for plants cultivated both on open land and in greenhouses (Altinok et al. 2018; Velarde-Félix et al. 2018).
Previous studies have reported seedling wilt disease affecting A. koa A. Gray in Hawaii caused by F. oxysporum f. sp. koae, f. sp. nov. (Gardner 1980; Dobbs et al. 2020). Furthermore, F. oxysporum was reported to have attacked A. nilotica (L.) P. J. H. Hurter & McNabb seedlings in the greenhouse of the Forest Research Institute, India (Kapoor et al. 2004). In Papua (Indonesia), F. oxysporum has been found to cause damping-off disease on A. mangium seedlings six days after germination (Widyastuti et al. 2013). This research aims to identify the pathogenic species causing wilt disease in advanced seedlings of A. mangium and the disease’s pathogenicity.
Materials and methods
Survey and sampling
Soil and diseased plant samples were collected from six commercial, company-owned acacia nurseries with a seedling wilt problem. The diseased plants showed initial chlorosis; later, the plants wilted and dried up. The symptoms started from the lower leaves, moving on to the upper leaves and the shoots. Samples showing wilting symptoms were collected and stored in a cool box. To determine soil infectivity, acacia seeds were sown on a seedbed, and to accelerate germination, they were first soaked in hot water (± 95 °C) and left to cool until they reached room temperature (Gardner 1980). Subsequently, the seeds were surface sterilized using 1% sodium hypochlorite for 15 min, and rinsed three times with sterile distilled water. They were then incubated for 48 h to accelerate the radicle development. The germinated seeds were planted on infested field nursery soil. The infected seedlings from the field and the infected nursery soil were sampled and the pathogen isolated from the plant tissue.
Fungal isolation
Fungi were isolated from the roots of plants growing in the field that showed symptoms of wilt and from soil infected with pathogens. The root samples were then washed under running water and the surface sterilized by dipping in a solution containing 1% sodium hypochlorite for 2 min, rinsing three times in distilled water, and drying on filter paper in a laminar airflow (Suwandi et al. 2012). They were then laid out on a Petri plate of 2% (w/v) agar and 0.1% streptomycin sulfate, and incubated for 48 h (Gardner 1980; Leslie and Summerell 2006; Suwandi et al. 2012). The mycelium from the root cuttings was transferred to PDA medium using the single hyphae method. The isolate results were used for further research.
Morphological identification
The initial identification was carried out based on Leslie and Summerell (2006). The observation of cultural characteristics and the morphology of the colony included growth rates and color pigments produced on PDA media. Asexual spores and other structures were observed on the growth produced on carnation leaf agar (CLA) medium. These were made under a light microscope (OLYMPUS CX 23) at 1000 × magnification with a camera (Optilab Advance Plus, Yogyakarta, Indonesia). Measurements were carried out on 100 spores of microconidia and macroconidia, and chlamydospores using Image Raster 3.0 software with magnification adjusted to a microscope.
Pathogenicity test
This test was carried out on A. mangium seedlings 30 days after sowing in plastic pots containing 200 g peat soil which had previously been sterilized. Fungal isolates were grown on a potato dextrose broth (PDB) medium by placing 5 × 5 mm agar pieces of the fungal colony in the broth. The cultures were incubated for three days using a shaker at a speed of 120 rpm to produce large quantities of conidia. This suspension was used as inoculum by pouring 1 × 106 cfu g−1 (colony forming unit/g) on a soil medium while the uninoculated control was sterile distilled water. Each isolate was inoculated into the soil of 10 test plants and the experiment repeated once. Disease incidence was counted as the number of diseased plants out of 10. The severity of the disease was calculated for each seedling using a score of 0 − 4, where 0 = no disease/healthy seedling, 1 = yellow leaves, 2 = yellow leaves and slightly wilted, 3 = severe wilt, and 4 = dead seedling. The plants were observed—over 30 days after inoculation. The difference in disease severity and the area under the disease progress curve (AUDPC) between isolates was calculated through ANOVA and Tukey’s HSD test. An analysis was performed using the SAS university edition software package.
Molecular identification
Seven representative fungal isolates with distinct morphological characteristics were selected and grown in a cultivation bottle containing 50 mL sterile PDB (200 g potato; 20 g glucose; 1 L distilled water). Sections of 3 − 4 day-old cultures on the PDA medium, measuring 5 mm × 5 mm, were placed on a PDB liquid medium and incubated for 3 − 4 additional days at room temperature. The fungal mycelium was harvested using vacuum filtration and frozen. DNA was extracted using the YeaStar Genomic DNA Kit (Zymo Research Corporation, Irvine, CA, USA) following manufacturer’s instructions. Its concentration and quality were determined by spectrophotometry using a NanoDrop Spectrophotometry ND-1000 (NanoDrop Technologies, Montchanin, DE, U.S.A.) and stored at − 20 °C until used. The translation elongation factor 1-α (tef1) was amplified using primers EF1 (forward: 5′-ATGGGTAAGGAAGACAAGAC-3′) and EF2 (reverse: 5′-GGAAGTACCAGTGATCATGTT-3′) (O’Donnell et al. 1998). PCR was carried out in 50 µL of the reaction mixture containing 20 µL Master Mix (Eppendorf, Germany) (1.25 GoTaq DNA polymerase, 0.2 µM of each dNTP, 2 × PCR buffer), 1 µL of each primer, and 2 µL of DNA template. The amplification was performed using a PCR Cycler Termal C1000 Touch ™ (Bio-rad, USA). The initial denaturation was performed for 2 min at 95 °C followed by 30 cycles for 20 s at 95 °C, annealing for 40 s at 58 °C, and extension for 1 min at 65 °C, with a final elongation step of 5 min at 65 °C (Suwandi et al. 2012). Sequences of the PCR products were analyzed at 1st BASE, Co., Ltd., Kuala Lumpur, Malaysia. The sequence data obtained was determined by comparing the GenBank (http://www.ncbi.nlm.nih.gov) and FUSARIUM-ID databases. To determine the genetic relatedness of F. oxysporum from A. mangium with the known Fusarium population, the tef1 sequences were aligned using Clustal-W in MEGA7 and maximum parsimony (MP) analyses were performed. There was a total of 577 positions in the final dataset. All positions containing gaps and missing data were eliminated. The MP tree was obtained using the subtree pruning re-grafting algorithm (Nei and Kumar 2000) under MEGA7 (Kumar et al. 2016).
Results
Disease symptoms and wilt incidences
Surveys on seedling wilt were carried out in six acacia nurseries of commercial companies; five sites in the Air Sugihan area and one in Lebong Hitam (Table 1). The incidence of disease varied between locations from 6.0% up to 36.9%. The early symptoms of seedling wilt started as the lower leaves turned yellow, then black, dried out, fell and the plant died. Some symptoms began with yellowish discoloration of the leaves, which then wilted, and some had no discoloration but immediately began with wilting (Fig. 1). Observations were made by planting acacia seedlings on used soil medium from the commercial nursery, and the results showed that 56.25% of seedlings were attacked. In the commercial nurseries, this disease attacks seedlings at an average age of more than 1 month after germination and before the formation of phyllodes or modified petioles or stems.
Morphological characteristics
Fifteen isolates similar to F. oxysporum were taken from the root tissues of diseased plants (Table 2), and grown on PDA and CLA media (water agar medium with carnation leaf pieces) at 27 °C. The PDA cultures produced purple, pale purple and reddish-pink pigments with air hyphae (Fig. 2). These isolates showed varied growth rates of the colony, with the fastest on BF05 and FF15 isolates (11.4 mm day−1). BF06 had the slowest growth rate (7.5 mm day−1) (Fig. 3). They all produced numerous microconidia on the false heads of monophialides. Hyaline microconidia are oval, elliptical, reniform to allantoid with an average size of 5.5 ± 1.0 μm × 2.8 ± 0.5 µm, to 11.8 ± 4.1 μm × 3.0 ± 0.3 µm, and have zero to one septum but generally zero. The hyaline macroconidia, being sickle-shaped, have an average size of 33.6 ± 5.4 × 3.9 ± 0.5 µm, to 39.7 ± 5.9 × 3.8 ± 0.4 µm, and have three to seven septa but usually three. The chlamydospores produced singly or in pairs at the terminal/intercalary have an average diameter of 6.4 ± 0.6 µm to 10.0 ± 2.5 µm (Table 3). Based on the morphological characteristics of the PDA and CLA media, all isolates were in accordance with the F. oxysporum as described by Leslie and Summerell (2006).
Molecular characteristics
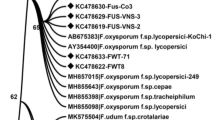
Molecular identification by sequencing the tef1 gene was amplified using primers EF1 and EF2 against seven selected isolates. The sequence data was determined by a comparison with those in Genbank through the BLAST and FUSARIUM-ID programs. Based on this, the sequences were confirmed as F. oxysporum with 84.9 − 100% similarity to Genbank and 97.3 − 99.7% with the FUSARIUM-ID. Phylogenetic analysis (consistency index 0.8, retention index 0.9 and the composite index 0.8) grouped all Fusarium isolates within the F. oxysporum species complex. Isolates were clustered within four clades (Fig. 4). The first clade comprised BF05 and EF14, and F. elaeidis (MH484961.1) from Elaeis spp. The second clade consisted of single isolates from A. mangium (DF11), F. oxysporum (C009W and C010W) from Cucumis melo, and F. triseptatum (MH484964.1) from Ipomoea batatas. The third clade contained AF01, AF03 and BF07 along with BTGN4 (F. oxysporum from Sanseviera trifasciata) and FO393 (F. oxysporum from Vanilla planifolia). The fourth included DF12 and an isolate of F. oxysporum from Musa sp. Tef1 sequences of isolates within third and fourth clade were separated from all 15 cryptic taxa of F. oxysporum species complex as described by Lombard et al. (2019).
One out of the nine most parsimonious trees showing the genetic relatedness, represented by the tef1 sequences, between Fusarium oxysporum from Acacia mangium-(in bold), closely related Fusarium oxysporum isolates, and other species of Fusarium isolates. The strain numbers, host species and countries of origin are given with the representative isolates. A bootstrap test with 1000 replicates produced a bootstrap value greater than 50%, shown at the appropriate node. The genetic distance is indicated by the scale bar
Pathogenicity tests
Fusaria, isolated from diseased plant tissue, were tested for their pathogenicity in 30-day-old A. mangium seedlings. The results showed that symptom development began with yellowing of the lower leaves or those closest to the base of the stem, then their wilting, curving upwards, turning brown to black, drying and falling from the plant. This progresses to the top of the plant, causing it to wither and die. In addition, infected plants show symptoms of stunted growth. The first symptoms appeared 7–14 days after inoculation and were followed by total plant collapse within 1–10 days. However, some plants showed complete wilting immediately without any initial symptoms (Fig. 5). The control plants were healthy and did not show any wilting symptoms. All the isolates were able to infect plants and disease incidence and severity were significantly higher compared to the control. The AF02, BF06, CF10, DF11, DF12 and DF13 isolates caused disease severity and progress and their AUDPC values were significantly higher than other isolates and the control (Table 4). With regards to disease incidence, the Fusarium isolates had a high variation in percentage of disease incidence, ranging from 50 to 100%. These isolates also induced wilting severities of 1.2 − 3.2. The highest disease incidence was caused by isolate BF06 (100%), while the highest disease severity was caused by isolate DF11 (3.2). Based on disease severity, the isolates were grouped into three categories of virulence: high (score 2.0 − 4.0), moderate (score 1.2 − 2.0), and low score (0 − 1.2) virulence (Dubey et al. 2010). BF08, AF01, AF02, AF04, CF10, DF12, DF13, BF06, and DF11 were included in the high virulence group (2.1 − 3.2), while FF15, BF09, BF07, AF03, and BF05 were in the moderate (1.6 − 2.0), and only EF14 had a low score. We observed that this pathogen causes vascular disease, where the pathogen could be isolated from the basal stem up to the shoots. To confirm the Koch’s Postulates test, the pathogens were all re-isolated (100%) from infected plants and it was confirmed that the morphological characters of the pathogen were the same as the inoculated Fusarium isolates. The pathogen was not isolated from the uninoculated control plants.
Discussion
This research reports that F. oxysporum has been identified for the first time as a causative agent for A. mangium seedling wilt in South Sumatra, Indonesia. According to previous studies, F. oxysporum was reported as the cause of seedling wilt in A. koa in Hawaii (Gardner 1980) and A. nilotica in India (Kapoor et al. 2004). In Indonesia, this pathogen was reported to attack germinated acacia seeds, resulting in damping-off, and the identification of this was made solely on morphological characters (Widyastuti et al. 2013). Our research confirms that F. oxysporum, identified by morphological and molecular methods, causes vascular wilt disease in advanced seedling stages. Another study by Luo and Yu (2020) has demonstrated that F. oxysporum causes damping-off on Pinus massoniana Lamb.
The pathogenicity test confirmed that all the isolates were able to develop wilt symptoms with different disease incidence and severity, some reaching 90−100% and scoring 2.1−3.2, respectively. Other studies have reported that F. oxysporum causes wilt diseases on A. nilotica in India with diseases severity of 16.9% (Kapoor et al. 2004), on A. koa in Hawaii with 85.0% severity (Gardner 1980), and damping-off on A. mangium in Papua, Indonesia with unknown disease severity (Widyastuti et al. 2013).
The results here have also shown that F. oxysporum is a pathogen causing vascular wilt in A. mangium seedlings, which is evident in the isolation in the shoots. The pathogens infect the roots, then enter and multiply along the xylem vessels, and are translocated to the shoots through water movement. Browning in the xylem tissue, which is typical of vascular wilt disease (Meena and Roy 2020), was also exhibited in the inoculated plants.
F. oxysporum was determined based on morphological identification, both visually and microscopically. Visually, the fungus produces pale purple to pale pink pigments on a PDA medium. Microscopically, the shape of its reproductive structure is the same as F. oxysporum, generally exhibiting a short monophialide (Leslie and Summerell 2006). The analysis of the tef1 gene sequences confirmed that the disease-causing pathogenic species was F. oxysporum. These showed that F. oxysporum, which causes acacia seedlings to wilt, is the most similar to the F. oxysporum species complex from Elaeis sp. (F. elaeidis) (Lombard et al. 2019), S. trifasciata (Kee et al. 2020), V. planifolia (Koyyappurath et al. 2016), C. melo (Bakar and Mohd 2019).
Field observation of the disease in the commercial nursery showed that about 36.9% of plants died through Fusarium wilt. This attack caused large economic losses because these nurseries provide seedlings for commercial gardening in South Sumatra over 1,324,653 hectares. The high disease incidence in the field was possibly caused by the previous use of seedlings infected with the pathogens, which led to rapid development and accumulation through the nursery cycle. This is evident in the high disease incidence on soil media. It is likely that clonal dispersion occurred via the nursery medium to the commercial field nursery as shown by isolates within the first clade of the tef1 sequences originating from separate locations. Jiménez-Díaz et al. (2015) reported that the main source of primary inoculum that causes Fusarium wilt comes from infected soil and that pathogens can survive a long periods in the soil, and in other plant debris (Postic et al. 2012; Altinok 2013).
Conclusions
This study is the first report of F. oxysporum as a causal agent of A. mangium seedling wilt in South Sumatra, Indonesia. The pathogen was confirmed through morphological and tef1 gene sequencing and Koch’s postulate. The main source of a primary inoculum which causes Fusarium wilt disease in South Sumatra comes from seedling soils contaminated with pathogens.
References
Altinok HH (2013) Fusarium species isolated from common weeds in eggplant fields and symptomless hosts of Fusarium oxysporum f. sp. melongenae in Turkey. J Phytopathol 161:335–340
Altinok HH, Can C, Altinok MA (2018) Characterization of Fusarium oxysporum f. sp. melongenae isolates from Turkey with ISSR markers and DNA sequence analyses. Eur J Plant Pathol 150:609–621
Bakar MA, Mohd M (2019) Characterization of Fusarium oxysporum isolated from agricultural and non-agricultural ecosystems in Malaysia. Unpublished
Bayona LG, Grajales A, Cardenas ME, Sierra R, Lozano G, Garavito MF, Restrepo S (2011) Isolation and characterization of two strains of Fusarium oxysporum causing potato dry rot in Solanum tuberosum in Colombia. Rev Iberoam Micol 28(4):166–172
Dobbs JT, Kim MS, Dudley NS, Klopfenstein NB, Yeh A, Hauff RD, Stewart JE (2020) Whole genome analysis of the koa wilt pathogen (Fusarium oxysporum f. sp. koae) and the development of molecular tools for early detection and monitoring. BMC Genom 21:1–15
Dubey SC, Singh SR, Singh B (2010) Morphological and pathogenic variability of Indian isolates of Fusarium oxysporum f. sp. ciceris. Arch Phytopathol Pflanzenschutz 43(2):174–190
Edel-Hermann V, Lecomte C (2019) Current status of Fusarium oxysporum formae speciales and races. J Phytopathol 109:512–530
Gardner DE (1980) Acacia koa seedling wilt caused by Fusarium oxysporum f. sp. koae, f. sp. nov. J Phytopathol 70(7):594–597
Gordon TR (2017) Fusarium oxysporum and the Fusarium Wilt Syndrome. Annu Rev Phytopathol 55:23–39
Hedge M, Palanisamy K, Yi JS (2013) Acacia mangium Willd. A fast growing tree for tropical plantations. J For Sci 29(1): 1–14.
Jiménez-Díaz RM, Castillo P, MdelM J-G, Landa BB, Navas-Cortés JA (2015) Fusarium wilt of chickpeas: biology, ecology and management. Crop Prot 73:16–27
Kapoor S, Harsh NSK, Sharma SK (2004) A new wilt disease of Acacia nilotica caused by Fusarium oxysporum. J Trop for Sci 16(4):453–462
Kee YJ, Zakaria L, Mohd MH (2020) Morphology, phylogeny and pathogenicity of Fusarium species from Sansevieria trifasciata in Malaysia. Plant Pathol 69(3):442–454
Koyyappurath S, Atuahiva T, Le Guen R, Batina H, Le Squin S, Gautheron N, Grisoni M (2016) Fusarium oxysporum f. sp. radicis-vanillae is the causal agent of root and stem rot of vanilla. Plant Pathol 65(4):612–625
Kumar S, Stecher G, Tamura K (2016) MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33(7):1870–1874
Leslie JF, Summerell BA (2006) The fusarium laboratory manual. Blackwell Publishing, Oxford
Lombard L, Lamprecht SC, Crous PW (2019) Epitypification of Fusarium oxysporum–—clearing the taxonomic chaos. Persoonia 43:1–15
Luo X, Yu C (2020) First report of damping-off disease caused by Fusarium oxysporum in Pinus massoniana in China. J Plant Dis Prot 127:401–409
Meena RP, Roy S (2020) Morphological and molecular characterization of Fusarium sp. causing wilt disease of isabgol (Plantago ovata Forsk.) and its management strategies. J Appl Res Med Aromat Plants 100244
Nei M, Kumar S (2000) Molecular evolution and phylogenetics. Oxford University Press, New York
O’Donnell K, Kistler HC, Cigernik E, Ploetz RC (1998) Multiple evolutionary origins of the fungus causing Panama disease of banana: concordant evidence from nuclear and mitochondrial gene genealogies. Proc Natl Sci USA 95:2044–2049
Orr R, Nelson PN (2018) Impacts of soil abiotic attributes on Fusarium wilt, focusing on bananas. Appl Soil Ecol 132:20–33
Postic J, Cosic J, Vrandecic K, Jurkovic D, Saleh AA, Leslie JF (2012) Diversity of Fusarium species isolated from weeds and plant debris in Croatia. J Phytopathol 160:76–81
Statistics Indonesia (2018) Timber culture establishment. BPS-Statistics Indonesia, Jakarta
Sun FF, Sun SL, Zhu L, Duan CX, Zhu ZD (2019) Confirmation of Fusarium oxysporum as a causal agent of mung bean wilt in China. Crop Prot 117:77–85
Suwandi AS, Kondo N (2012) Common spear rot of oil palm in Indonesia. Plant Dis 96(4):537–543
Velarde-Félix S, Garzón-Tiznado JA, Hernández-Verdugo S, López-Orona CA, Retes-Manjarrez JE (2018) Occurrence of Fusarium oxysporum causing wilt on pepper in Mexico. Can J Plant Pathol 40(2):238–247
Widyastuti SM, Tasik S, Harjono, (2013) Infection process of Fusarium oxysporum fungus: a cause of damping-off on Acacia mangium’s seedlings. Agrivita 35(2):110c118
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Project funding: The work was supported by the Directorate General of Research and Development, Ministry of Research, Technology and Higher Education through the PMDSU scholarship 2020−2021 according to the Director of Research and Community Service, Directorate of Research and Community Service, chaired by Ahmad Muslim number 0124/UN9/ SB3.LP2M.PT/2020.
The online version is available at http://www.springerlink.com.
Corresponding editor: Tao Xu.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Soleha, S., Muslim, A., Suwandi, S. et al. The identification and pathogenicity of Fusarium oxysporum causing acacia seedling wilt disease. J. For. Res. 33, 711–719 (2022). https://doi.org/10.1007/s11676-021-01355-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11676-021-01355-3