Abstract
Forests in Northeast China in the Greater and Lesser Khingan Mountains (GKM and LKM) account for nearly 1/3 of the total state-owned forests in the country. Regional and historical comparisons of forest plants and macrofungi will favor biological conservation, forest management and economic development. A total of 1067 sampling plots were surveyed on forest composition and structure, with a macrofungi survey at Liangshui and Huzhong Nature Reserves in the center of two regions. Regional and historical differences of these parameters were analyzed with a redundancy ordination of their complex associations. There were 61–76 families, 189–196 genera, and 369–384 species, which was only 1/3 of the historical records. The same dominant species were larch and birch with Korean pine (a climax species) less as expected from past surveys in the LKM. Shrub and herb species were different in the two regions, as expected from historical records. There was 10–50% lower species diversity (except for herb evenness), but 1.8- to 4-time higher macrofungi diversity in the GKM. Compared with the LKM, both tree heights and macrofungi density were higher. Nevertheless, current heights averaging 10 m are half of historical records (> 20 m in the 1960s). Edible macrofungi were the highest proportion in both regions, about twice that of other fungal groups, having important roles in the local economy. A major factor explaining plant diversity variations in both regions was herb cover, followed by shrubs in the GKM and herb-dominant species in the LKM. Factors responsible for macrofungi variations were tree density and shrub height. Vaccinium vitis-idaea and Larix gmelinii in the GKM but tree size and diversity were important factors in the LKM. Our findings highlighted large spatial and historical differences between the GKM and LKM in plant-macrofungal composition, forest structure, and their complex associations, which will favor precise conservation and management of forest resources in two region in the future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Biodiversity is one of the most critical factors determining ecosystem functions and services in maintaining energy flow, purifying the environment, and regulating microclimate (Wang 2019; Yang et al. 2019). For plants, detailed records of species composition of trees, shrubs and herbs in sampling plots is fundamental for the study of diversity, evenness and richness, and the survey of community characteristics related to individual size and density is the basis for understanding the relationship between forest structure and ecosystem function (Wang et al. 2020a, b). There is less research on macrofungi compared to plants, and the classification of species and their functional types (edibility, medicinal and toxicity) is a major interest of numerous scholars (Mao 2000; Liu 2004; Bau and Li 2010; Deng 2010; Wu et al. 2019). China’s forest resources are in a critical period of transition from insufficient quantities and low quality to a high quality and rapid accumulation (Qian 2014; Yang 2018). Comparison of the current status and historical records may help to identify steps to improve plant and fungal resources, their conservation, and management for enabling this transition.
Complex decoupling among species diversity, species dominance, forest structure and geo-climatic conditions is crucial for conservation and management practices, and inclusion of forest characteristics at different vertical layers, including taxonomic groups, plant size and density, will help the evaluation of forest resources. The dominance of species (importance value and abundance), species diversity (richness, Simpson and Shannon–Wiener index, evenness index), combined with forest characteristics are required for understanding diversity formation mechanisms (Song and Liu 1995; Ali et al. 2018). A statistical method such as redundancy analysis (RDA) is beneficial to find statistically significant factors responsible for biodiversity variations (Gu et al. 2020; Wang et al. 2020a, b).
Forests in the Greater and Lesser Khingan Mountains (GKM and LKM), covering approximately 0.4 million km2, are important state-owned forests and classified as priority areas of the Natural Forest Protection Program (NFPP), a program of forest protection first established in 2000. Several studies using relatively small numbers of sample plot (< 100) were on vegetation resources (Han et al. 2004; Xie 2017; Yang et al. 2017a; Sun et al. 2020), species diversity (Zhou et al. 1998; Mao and Zhu 2006; Zhang et al. 2007) and structure (Xu and Jin 2012; Ren et al. 2013; Yang 2019), concentrated mainly in a single protected area or region. Large- scale integrated, comparative analysis of macrofungi and plant resources has not reported for the GKM and LKM. NFPP has currently banned timber harvesting, and rational utilization of understory plant and macrofungal resources has become a major income for millions of local people (Bau and Li 2010; Wang et al. 2012; Mysological-Society-of-China 2016; Wu et al. 2019). Detailed comparison of forest sources on plants and macrofungi, together with timber-related data, is necessary for the implementation of forest policies according to regional differences during the second stage of NFPP (http://www.forestry.gov.cn/).
In this study, we hypothesized that sharp regional and historical differences in plant and macrofungal composition, and forest structure, as well as their different associations require different measures for biodiversity conservation and management in the GKM and LKM. We were particularly interested in exploring the following questions: (1) What are the differences between dominant species, community structure and species diversity of plants and macrofungi in the two regions and the differences between today and in the past; (2) What is the association between species diversity and abundance and forest structure; (3) What is the association between macrofungi diversity and abundance, forest structure, and plant species diversity.
Materials and methods
Experimental design and plot census
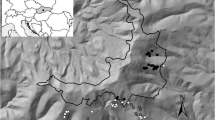
A total of 1067 plots (469 plots in the GKM, 598 in the LKM) were surveyed for tree, shrub and herb species. Plot sizes for the tree and macrofungi components were 30 m × 30 m. In each plot, ten 2 m × 2 m shrub subplots and ten 1 m × 1 m herb subplots were established. Elevation, latitude and longitude were recorded for each plot. (Fig. 1, Table 1). Species names, size of tree, shrub and herb layers, plant density (number, cover) and individual heights and diameters were recorded. Altitude, slope aspect (sunny, shade and partial sunshade), position (upper, middle, bottom or flat), and gradient in degrees were recorded.
A total of 146 plots for macrofungi were surveyed in Liangshui (center of the LKM) and Huzhong (center of the GKM) in the National Nature Reserves. In each 30 m × 30 m plot, species name, the total number of each macrofungi, and growing habitats (soil, litter, living tree and deadwood) were recorded three times by cross-line checking. Macrofungi were identified by traditional phenology with the help of microscopic observation. For phenological observation, visual identification or the use of magnifying lens were used to check e color, shape, ancillary features of the hypophysial, pileus, mediotrastum, collarium, stipe, volva and rhizomorph on-site and also a minimum of five digital photos were taken for later re-checks. For some macrofungi, spore prints from the sporocarp were also collected; Melzer's reagent was used to identify fungi through amyloid (from blue to black) and dextrinoid (brown to red-brown) color reaction. All photos were taken from different angles and simple anatomy, named according to sample number for convenient later laboratory recognition. The identification in the field and in the laboratory was carried out by referring to relevant literature (Huang 1998; Mao 2000; Liu 2004; Xiang 2005; Yu et al. 2005; Chen et al. 2013; Nature-Museum-Editorial-Board 2014; Shao and Xiang 2017). A re-confirmation of identification was also achieved with the assistance of a noted macrofungi expert, Prof. Cunti Xiang, retired from NEFU with two macrofungal books (Shao and Xiang 1997; Xiang 2005). The macrofungi were also checked in the 10th edition of the fungus dictionary (Kirk et al. 2008), the IndexFungorum online database (www.indexfungorum.org and www.speciesfungorum.org).
Dominant species abundance and diversity
Dominance at different taxonomic levels was recognized by the importance value (IV) as an average of relative coverage, frequency and abundance. Relative abundance was calculated by the individual number of species divided by the total individual number in the plot. According to the IV, the top five species, genera, and families in the tree, shrub and herb layers were recognized as dominant in the two regions. The calculations of IV are listed as Eqs. 1–4 in Table 2. After identification of dominance, the relative abundance in species, genera and families in each plot were also compared to find differences in vegetation in the GKM and LKM.
Four indices of species diversity were calculated from the field data as richness, Shannon–Wiener and Simpson diversity and evenness (Eqs. 5–8, Table 2), which have been used widely in previous reports (Ma et al. 1995). Plant species names were confirmed from the Flora of China (http://www.iplant.cn/frps), the Chinese Field Herbarium (CFH, http://www.cfh.ac.cn/spdb/spsearch.aspx), and the Flora of Woody Plants and Herbs in Northeast China (Liu 1955; Fu 2004). At the first appearance of Latin names of plant species, the authority names were listed; while thereafter, abbreviated names with genus and given name were used for simplifying the description of the result.
Forest structure
In each plot, all trees > 2 cm in basal diameter were measured: for trees, diameter at breast height (DBH), height (Th), and density (Td); for shrubs, density (Sd), height (Sh) and cover (Sc), and for herbs, cover of each species (Hc) and height (Hh). Shrubs and herb cover were determined as the percentage of area covered by the species to the total area. Tree and shrub densities were calculated as the number of individuals divided by the plot area. All these structural parameters were averaged as a plot mean value. Equations are listed in Table 2.
Macrofungi traits
After species identification, all macrofungi were divided into five utilization-related functional groups: edible, medicinal, toxic, wood-rot, as well as an unknown function, and four habitat-related functional groups (living tree, deadwood, soil-based, and litter habitat). These function groups were used to analyze the functional changes of macrofungi from habitat and utilization viewpoints (macrofungi names in Table S1).
The diversity of macrofungi was calculated following (Bau and Li 2000).
where Ai, Bi, Ci are relative densities (species i density/total density of the plot), relative abundance (species i number/total macrofungi number in the plot) and relative frequency (species i frequency in total surveyed plots/the number of plots). S and N are the total number of species and macrofungi numbers in the ith plot.
To determine compositional changes, the appearances of different taxon (total sum and plot average), and their relative abundance were computed. The relative abundance of different taxon in different regions were calculated as the percentage of dominant species, genera, family and order to total macrofungi observations in this study.
Data processing
A one-way analysis of variance (ANOVA) by SPSS 22.0 (SPSS, USA) was used to compare the significance of the two regional differences of dominant families, genera and species, structural features and species diversity of plants and macrofungi, and different functions (edible, medicinal, toxic, wood-rot, and unknown function macrofungi) and habitat (living tree, deadwood, soil-based and litter-habitat macrofungi). To find the relative change, a GKM/LKM ratio was calculated for all parameters. A statistically significant larger ratio indicated either that large regional differences existed or there were no differences.
Redundancy analysis (RDA) was carried out to explore the effect of species abundance and structural characteristics on diversity and the coupling relationship of macrofungi (different habitats, functional groups and diversity indices), forest characteristics (dominant species abundance and structural features of tree, shrub and herb layers) in the two regions. A comparison with detrended correspondence analysis (DCA) is listed in Table S2. Significant factors responsible for plant species diversity and macrofungi variations were identified under simple term and conditional term effects (excluding multicollinearity among factors). RDA analysis was performed by Canoco 5 (Biometrics Ltd., Plant Research International, the Netherland).
Results
Differences in the composition of plant resources
The number of families, genera and species in both regions were similar, 61 families, 189 genera and 384 species of plants in the GKM, among them, 8 families, 17 genera and 29 species were in the tree layer, 12 families, 20 genera and 41 species in the shrub layer, and 49 families, 159 genera, 314 species in the herb layer. There were 76 families, 196 genera and 369 species in the LKM, including 12 families, 21 genera and 45 species in the canopy layer, 18 families, 26 genera and 42 species in the shrub layer, and 60 families, 153 genera and 282 species in the herb layer (Tables S3 and S4).
Importance value (IV)-based relative abundance data showed that dominant families, genera and species of trees, shrubs and herbs were different in the GKM and LKM, especially for herbs and shrubs. The top four tree families, Pinaceae, Betulaceae, Salicaceae and Fagaceae, were the same in both regions, accounting for 98.9% of IV in the GKM and 82.8% in the LKM (Fig. 2a and b). The dominant genera of the two regions, Larix, Betula, Populus, Pinus, and Quercus were identical and accounting for 95.6% in the GKM and 74.6% in the LKM (Fig. 2c and d). Dominant tree species in the GKM included Larix gmelinii (Ruprecht) Kuzeneva, Betula platyphylla Suk, Populus davidiana Dode, Quercus mongolica Fischer ex Ledebour and Pinus sylvestris var. mongolica Litv. (91.1% of total IV), with the first four were the same, followed by Pinus koraiensis Siebold et Zuccarini in the LKM (Fig. 2e and f).
Differences in the relative abundance in IV of tree families, genera and species in the two regions. Notes Relative abundance was based on the proportion of importance value (IV) for each species, genus, and family; names of the top five species in relative abundance in IV are highlighted in the pie chart and total ranks listed in Tables S3 and S4; relative abundance of each parameter = IV of the parameter/total IV sum of all parameters in this layer
The most abundant shrub family in the GKM was Ericaceae (41.7% of IV, 2.3% in LKM), followed by Rosaceae and Betulaceae (the dominant top two in the LKM) (Fig. 3a and b). The dominant shrub genera in the GKM were Vaccinium, Spiraea, Corylus, Rhododendron and Betula (70.5% of IV sum). Corylus and Spiraea were also found in the LKM (58.3% of IV), followed by Sorbaria, Rosa and Lonicera (6.5–8.0% of IV) (Fig. 3c and d). The dominant shrub species were Vaccinium vitis-idaea Linn, Corylus heterophylla Fisch. ex Trautv, Rhododendron dauricum Linn, Spiraea media Schmidt. and Ledum palustre L. (54.9% of IV) in the GKM. In the LKM, the two most abundant species were Spiraea salicifolia L. and Corylus mandshurica Maxim. (48.4% of IV), followed by Corylus heterophylla, Sorbaria sorbifolia (L.) A. Br. and Rosa davurica Pall. (each IV was nearly 8.0%) (Fig. 3e and f).
The dominant herb families in the GKM were Cyperaceae, Poaceae, Rosaceae, Asteraceae and Asparagaceae (58.7% of IV), but the ranking was different in the LKM (Fig. 4a and b). Dominant herb genera in the GKM were Carex, Deyeuxia, Sanguisorba, Vicia and Pyrola (nearly half of the IV), the first two in the GKM along with Caldesia, Filipendula and Equisetum played an important role in the LKM (Fig. 4c and d). Dominant herb species in the GKM included Deyeuxia purpurea (Trinius) Kunth, Carex callitrichos V. Krecz, Sanguisorba officinalis L., Pyrola rotundifolia Linn. and Fragaria orientalis Lozinsk. (30.9% of IV); only the first principal species was the same, followed by Caldesia parnassifolia (Bassi ex L.) Par, Filipendula palmate (Pall.) Maxim, Carex dispalata Boott and Equisetum sylvaticum L. in the LKM (Fig. 4e and f).
The difference in species abundance showed that, in the tree layer, Larix gmelini, and Betula platyphylla had the largest proportions in both regions. Larix gmelini in the GKM was twice that in the LKM (p < 0.01). The abundance of Salicaceae (Populus and Populus davidiana) and Rosaceae (p < 0.01) was 1.3–2.1 times higher in the GKM, while Fagaceae (Quercus mongolica) in the GKM was half that of the LKM; Pinus koraiensis was only found in the LKM (p < 0.01, Fig. 5).
Compared with the LKM, the abundance of some shrub species was significantly higher in the GKM than in the LKM, such as Ericaceae – Vaccinium and Vaccinium vitis-idaea increased as much as 12-fold over the LKM; 13 times higher of Fabaceae, almost 2–10 times higher of Rhododendron (Rhododendron dauricum) and Ledum palustre in the GKM. In contrast, Betulaceae and Corylus were half of the LKM. The number of Spiraea salicifolia, Rosa davurica, Sorbaria sorbifolia and Corylus mandshurica were 1/10 to half of the numbers in the LKM. The abundance of Caprifoliaceae and Lonicera in the LKM was much more than in the GKM (Fig. 6).
Herb families, genera and species such as Rosaceae, Sanguisorba and Sanguisorba officinalis, as well as Fragaria orientalis in the GKM were 3.4–5.1 times higher than in the LKM. Pyrola and Pyrola rotundifolia were 6.3-fold higher but Filipendula and Filipendula palmata were only 1/5–1/3 in the LKM. Fewer Carex dispalata and Alismataceae, Caldesia, Caldesia parnassifolia were also present in the LKM (Fig. 7).
Differences in the composition of macrofungi
A total of 207 macrofungi spp. belonging to 24 families and 7 orders were found in the GKM with 141 species of 32 families and 10 orders in the LKM (Table S3 and S4).
The order Agaricales had the largest percentage of macrofungi in the two regions but was more abundant in the GKM compared to the LKM (56.5% vs 40.0%). The most abundant family was Tricholomataceae in both regions (22.9–23.2%), followed by Russulaceae in the GKM (14.8%) and Polyporaceae in the LKM (18.8%). The major species in the GKM were Cortinarius tenuipes, Xeromphalina campanell, Cortinarius croceofolius, Coriolus hirsutus and Xerocomus badius; Fomes fomentarius and Collybia acervata were relatively abundant (4%) in the LKM (Fig. 8).
From the perspective of the functional groups in the two regions, edible macrofungi accounted for the largest proportion (2–22 times higher than other groups), followed by medicinal macrofungi (1.5–10 times higher). The abundance of all functional groups in the GKM was higher than in the LKM, 2–4 times higher of toxic, edible and wood-rod macrofungi in the GKM, but medicinal macrofungi were similar in the two regions. From the statistics of habitat, soil-based macrofungi accounted for the largest proportion in the GKM (5–21 times higher). The two-region comparison showed a higher abundance of different habitat fungi in the GKM, especially soil-based and deadwood macrofungi were 1.7–8.5 times higher than in the LKM (p < 0.05, Table 3).
Differences in plant and macrofungi communities
Forest community characteristics in the two regions were significantly different (p < 0.05), and the average change was macrofungi > shrub > herb > tree. There were denser forests (1.3 times higher in tree density, 8.2 times higher in shrub density, 2.1 times higher in herb coverage and 3.3 times higher in macrofungi density); smaller trees (heights and DBH reduced 10–20%), smaller shrubs (height and cover reduced 60%) and shorter herbs (height decreased by 40%) in the GKM compared with those in the LKM (Table 4).
Differences in plant and macrofungi species diversity
Species diversity in the two regions was significantly different (p < 0.05 except for the Shannon–Wiener index and macrofungi evenness), and there was a larger change in macrofungi than for the vegetation. Compared with the LKM, macrofungi richness and diversity were 1.8–4 times higher but tree diversity, richness and evenness were 10–50% lower in the GKM. Shrub richness was similar in the two regions; other shrub indices decreased 40% in the GKM, while herb richness decreased 30% but was distributed more evenly in the GKM (1.2 times higher of evenness, Table 4).
Plant diversity association decoupling with species abundance and forest structure
Species diversity variations were similar (47.6% in the GKM and 50.8% in the LKM), and the most significant parameter was the same as for herb cover in both regions. In the GKM, herb cover was 22.7%, over twice as high as in the LKM (10%). Other factors were key species abundance of shrubs, trees (2–5%) and herbs (around 1%), structural features of tree DBH accounted for 1.1%, and shrub and herb layers accounted for 0.7–0.9% (p < 0.05). In the LKM, other important factors included the abundance of herbs, shrubs and trees (0.8–6.4%), and plant height and forest density (0.6–2.1%) (p < 0.05, Table 5).
In the GKM, the increase in species diversity of trees, shrubs and herbs coincided with the reduction of herb cover and shrub density, and increasing shrub cover and herb height. Larger trees, together with more abundant Quercus mongolica and Corylus heterophylla are usually accompanied by higher tree-herb diversity, while more Fragaria orientalis and less Vaccinium vitis-idaea and Deyeuxia purpurea reflected the higher shrub-herb diversity. In the LKM, shorter herbs, and less abundance of Deyeuxia purpurea, Caldesia parnassifolia, Larix gmelinii, Betula platyphylla and Spiraea salicifolia aligned with the higher diversity of all species. In addition, communities with lower herb cover, denser shrubs, and greater abundance of Corylus mandshurica, Quercus mongolica, Corylus heterophylla, Populus davidiana and Filipendula palmata had higher tree-herb diversity. In addition, shrub diversity increased with tree height and density (Fig. 9, Table 5).
RDA analysis of stand structure, dominant species and plant diversity variations in the GKM (a) and the LKM (b). Notes Factors in bold are the significant elements under conditional effects; species diversity abbreviation: T–H’ S–H’ and H–H’, Shannon–Wiener index of tree, shrub and herb layers; T–D, S–D and H–D, Simpson index of tree, shrub and herb layers; T–R, S–R and H–R, richness of tree, shrub and herb layers; T-Jsw, S-Jsw and H-Jsw, evenness index of tree, shrub and herb layers; stand structural features include Tdbh, diameter at breast height; Th, tree height; Td, tree density; Sh, shrub height; Sd, shrub density; Sc, shrub coverage; Hh, herb height; Hc, herb coverage. Dominant species abbreviation: PinsSylM., Pinus sylvestris var. mongolica; BetulaP, Betula platyphylla; PinusK., Pinus koraiensis; LarixG, Larix gmelinii; QuercusM, Quercus mongolica; PopulusD., Populus davidiana; SpiraeaM., Spiraea media; LedumPal.A., Ledum palustre; Rhodod.D, Rhododendron dauricum; VacciniumV, Vaccinium vitis-idaea; CorylusM, Corylus mandshurica; RosaD, Rosa davurica; SpiraeaS., Spiraea salicifolia; SorbariaS., Sorbaria sorbifolia; CorylusH., Corylus heterophylla; SanguisorbaO., Sanguisorba officinalis; FragariaO, Fragaria orientalis; CarexC, Carex callitrichos; PyrolaR., Pyrola rotundifolia; EquisetumS., Equisetum sylvaticum; FilipendulaP., Filipendula palmate; DeyeuxiaP., Deyeuxia purpurea; CaldesiaP., Caldesia parnassifolia; CarexD., Carex dispalata
Herb cover played the most important role in the increase in tree-herb diversity in the two regions. However, increased herb cover in the GKM was accompanied with the decrease of most of diversity indices; while in the LKM, Simpson, Shannon–wiener and evenness indices of shrubs increased with higher herb cover. In the GKM, shrub (10.2%) contributed 2–5 times more than tree and herb species for diversity variations (2–5.2%), while in the LKM, herb species were more common (15.2%) than tree and shrub species (total 9.2–9.5%). Less shrub density and higher herb heights led to higher species diversity in the GKM, but there was a lower tree-herb diversity in the LKM.
Macrofungi-related association decoupling with plant species and structural factors
The explanation for the effect on macrofungi variations were 56.2% and 44.5% in the GKM and LKM, respectively. Compared with the LKM, where only tree DBH and shrub species were significant factors, much more significant parameters were found in the GKM (Table 6).
In the GKM, macrofungi diversity and edible, soil-based macrofungi increased with more Vaccinium vitis-idaea and Larix gmelini and higher shrub cover, while macrofungi richness, medicinal, and wood rot macrofungi, living tree, deadwood and litter-based macrofungi increased with more abundant Spiraea media, higher shrub and herb cover and richness. All macrofungi of multiple functions, habitats and higher diversity increased with denser tree cover. In the LKM, the larger tree DBH and higher diversity but less Sorbaria sorbifolia led to more macrofungi of multiple function and habitat and higher diversity (Fig. 10, Table 6).
RDA analysis of forest plant features and macrofungi parameters in the GKM (a) and LKM (b). Notes Factors in bold are the significant elements under simple and conditional effects; plant features include stand structure, dominant species composition and diversity characteristics (abbreviations in Fig. 9); macrofungi parameters include macrofungi diversity: Shannon–Wiener (M–H’), Simpson (M–D), richness (M–R) and evenness index (M-Jsw); macrofungi functional groups include edible, medicine, toxic, wood rot and Unknown; habitat: living tree (Livetree), Deadwood, soil-based, litter-habitat (Litters) macrofungi, and macrofungi density (Md)
Discussion
Forest quality in two regions: present and historical differences
At the present time, the composition of the dominant tree species is similar in the two regions, but historically there were much greater differences (Table 7). At present, among the five dominant species in each region, four were the same historically in the canopy: Larix gmelinii, Betula platyphylla, Populus davidiana, and Quercus mongolica. However, of the top five shrub and herb species, only the shrub Corylus heterophylla and the herb Deyeuxia purpurea were the same in the two regions (Figs. 2, 3, 4). However, historically, Larix was dominant in the GKM and Pinus koraiensis broadleaf mixed forest was dominant climax vegetation in the LKM (Table 7). Due to extensive deforestation and poor management (Zhou 1997), the original climax vegetation in the LKM has changed with a sharp decline in Korean pine and numbers of other important species such as Franxinus mandshurica, Phellodendron amurense and Juglans mandshurica Maxim. (Zhou 1994). For the production of timber, a large area of secondary forest had a significant proportion of planted Larix gmelinii (Zhou et al. 1989). On heavily harvested sites, naturally regenerated Betula platyphylla was prevalent (Guan et al. 1997). In the history of the GKM, Larix gmelinii accounted for nearly 90% (Xu et al. 1997) in the 1950s, 66% in 1987 and 48% in 2003 (Liu 1990), birch increased from 12.6% in 1956 to 30.6% in 1987 and 36% in 2003 (Liu 1990; Chen et al. 2008) (Table 7). In this study, Betula platyphylla accounted for 20% with Larix gmelinii at 48%. Betula platyphylla is a pioneer species, increasing significantly after logging (Zhou et al. 1989). The abundance of Larix gmelinii in the GKM was twice that of the LKM (Fig. 5), and larch is the main climax species in the region (Zhou 1997). The homogenization of species in the two regions was related to the anthropogenic influence of afforestation and over-harvest secondary succession. The ecological risks should be highlighted for this homogenization (McKinney 2006).
Shrubs and herbs are different in the two regions, which is similar to their original history (Table 7). Vaccinium vitis-idaea prevailed in the GKM and Corylus spp. in the LKM (Zhou 1991) and are dominant today. However, the specific plant abundance declines should be noticed. For example, the percentage of Vaccinium uliginosum and Ledum palustre were sharply reduced in the GKM; herbs in the LKM were dominant by Deyeuxia purpurea, Carex spp. and Impatiens nolitangere, but the latter sharply decreased in our research (IV < 5%). In the GKM, some changes in herbs were found when compared with historical data (Table 7) (Zhou and Zhou 1985); medicinal and wild vegetable species became less dominant. Vaccinium vitis-idaea, Rhododendron dauricum, Ledum palustre accounted for 6%-21% in current shrub layer, and Sanguisorba officinalis, Pyrola rotundifolia and Convallaria majalis were 4%-5% in the present herb layer in the GKM. In the LKM, Sorbaria sorbifolia was 8% of the shrub layer, and Filipendula palmata for 9% of the herb layer. Their past abundance was much higher than it is today (Chinese-Herbal-Medicine-Teaching-and-Research-Office 1973; Nie 1980).
Species diversity in the LKM is higher than in the GKM today which is similar to historical records (Table 8). There was 10–50% less diversity of trees, shrubs and herbs, but herbs are distributed more evenly in the GKM. The total number of plant species recorded in this study (369–384) is much lower than the historical record (1003–1377 species, Table 8). This field study is part of a national project with national top-level scientists. However, in the 1950s–1970s, large-scale field studies with special emphasis on taxonomical identification were carried out by different teams, and the combined work of several taxonomists resulted in more detailed (http://www.iplant.cn/frps2019/). Another reason for species differences is due to the extinction of some species owing to excessive human disturbance (e.g., wild ginseng) and global climate changes (Pandolfi et al. 2020).
In conclusion, much denser forests of smaller sized trees were found in the GKM compared to the LKM, and forest layers were only half the heights in both regions compared with historical data (Table 9). In the case of tree height, annual rates of declining were 26.5 cm and 17.7 cm in the GKM and LKM; with DBH, annual rates of declining were 2.6 mm and 3.1 mm in the two regions, respectively (Table 9). The better forest structure usually means higher canopy, and good forest structure for forest ecological services is just as black soil thickness’s importance for farmland productivity. When considering black soil erosion crisis, the 0.3 cm year−1 erosion is usually mentioned (https://china.huanqiu.com/article/9CaKrnJBY9x). Our results show that forest degradation is 18–88-times higher than black soil degradation in this region (Table 9). Furthermore, our data also found that shrubs and herbs became much shorter than historically (Table 9). Over all, forest degradation related to forest structure in both regions needs more attention for the ecological well-being of the northeast forest belt.
Macrofungi in the two regions: differences and comparison
Compared with the LKM, macrofungal abundance was 1.3–10 times higher in the GKM except for living tree habitat macrofungi. This is possibly related to human impact differences. The population density in the GKM is 60 thousand people per km2, < 1/3 of the LKM (190 thousand people per km2). Historical records have shown a contrasting tendency with macrofungi species in the GKM lower than in the LKM (471 vs 578, Table 10). Macrofungi in LKM need to be protected in the future.
References have shown a similar species composition compared with our data. Different surveys in the GKM have reported 201–452 species, and a macrofungi list of 210–713 was found in the LKM (Table 10). In the Huzhong and Liangshui Reserves, not all previous lists were found in our research owing to lists of previous reports compiled by different scientists and sampled in different seasons. Until recently, the protection of natural reserves in China has been specified for threatened plants or animals (http://www.gov.cn/guoqing/2019-04/09/content_5380702.htm), without including macrofungi. Most previous studies have focused on macrofungi alone with multiple field surveys together with historical specimen identification. However, these studies did not include a regional comparison of macrofungi and plant diversity, and a detailed understanding of macrofungi and plant resources in taxon, structure and diversity as in this study, improves understanding of conservation of plant and macrofungi diversity.
Implications of this study
First of all, the understory contributes significantly forest community diversity, and more attention should be given to biodiversity conservation practices. Plant diversity, rather than macrofungi diversity, was easier explained by aboveground features. The understory forest economy of shrubs, herbs and macrofungal resources is developing rapidly (Bau et al. 2019). One concern is that this may lead to the over-exploitation of understory species, just as timber resources were historically over harvested (Zhou 1997). Our findings have clearly shown that over harvesting has resulted in changes in dominant tree species and the climax Korean pine has become a national species for protection. The same concern is that the extinction of understory species in the implementation of the NFPP. The conservation of understory resources should be considered in future forest resource management.
Secondly, association decoupling found differences between the two regions and provides guidance for improved and targeted management, which has been strongly encouraged by China’s central government (http://www.forestry.gov.cn/). In both regions, herb cover was the main explanation for diversity variations, indicating the importance of protecting the herb layer in in the two regions. However, a second layer of species composition was different in the two regions; i.e., for the GKM, it was Vaccinium vitis-idaea and Ledum palustre, and for LKM, it was Deyeuxia angustifolia, Carex dispalata, and Caldesia parnassifolia. Plant diversity conservation may benefit from the regulation of these species in both regions. For macrofungi management, higher density forests as well as more Vaccinium vitis-idaea and Larix gmelinii will benefit the diversified macrofungi in the GKM. However, in the LKM, higher tree DBH and diversity will accompany much richer macrofungi of function groups and habitat-related types.
Thirdly, some parameters are major indicators of plant and macrofungi diversity but differ in the two regions. Such indicators can facilitate the evaluation of natural conservation efforts. In recent years, some researchers have proposed to monitor changes in forest diversity with macrofungi as a substitute (Halme et al. 2017), and our data confirms that higher plant diversity is accompanied with higher macrofungi Simpson indices, more abundance of habitat-related macrofungi groups, and different utilization-related functional groups in the GKM. In the LKM, higher tree-shrub diversity but fewer herbs were aligned with richer and diverse macrofungi functional groups. This provides an indicator-related basis for monitoring changes in forest diversity using multiple methods, which is important for the further implementation of the Natural Forest Protection Program.
Conclusion
Although climax vegetation in the GKM (larch forests) and LKM (Korean pine-broadleaf mixed forests) is historically different, our data shows similar dominant species of Larix gmelinii and Betula platyphylla. Moreover, in the past half-century, the canopy height decreased from 0.2 to 0.3 cm year−1, and the shrub-herb layers by 0.4–0.8 cm year−1. Herb, shrub and macrofungi species in the GKM are considerably different from those in the LKM, which are similar to those historically. Complex associations between plant and macrofungi diversity and forest geographical location were observed, but significantly different explaining factors were found in these two regions. In both, the predominant explaining factor was herb cover, showing species importance for biodiversity conservation, and over-emphasis on understory utilization should be cautioned. Our findings provide detailed data for the evaluation of natural resources utilization and biodiversity, and also provide information for further implementations of the NFPP.
References
Ali A, Lin SL, He JK, Kong FM, Yu JH, Jiang HS (2018) Climate and soils determine aboveground biomass indirectly via species diversity and stand structural complexity in tropical forests. For Ecol Manage 432:823–831. https://doi.org/10.1016/j.foreco.2018.10.024
Bau T, Li Y (2000) Fungal community diversity in Daqinggou Nature Reserve. Acta Ecol Sin 20(6):986–991
Bau T, Li Y (2010) Wild eatible mushroom resources in Norhteastern China. In: Eatible mushroom symposium of China
Bau T, Wang X, Zhang P (2019) Floristic of agarics and boletus in the Greater and Lesser Khinggan Mountains. Biodivers Sci 27(8):867–873. https://doi.org/10.17520/biods.(2019)048
Bi XH, Wei X, Deng X (2006) Ecological distribution and resource evaluation of macrofungi in Natural Korean pine forests in Heilongjiang Province. For Sci Technol 31(5):26–30
Chen RJ, Ming HJ, Wang JN (2008) Research on forest structure characteristics in Inner Mongolia Daxing’an mountains. Inner Mong For Investig Des 31(1):56–58. https://doi.org/10.13387/j.cnki
Chen KL, Mao XL, Huang MD (2013) A guide to large antitumor medicinal fungi in China. Science Press, Beijing
Cheng GH (2018) Macrofungi diversity of Shengshan National Natural Reserve. Jilin Agriculture University, Changchun, Heilongjiang Province
Chinese Herbal Medicine Teaching and Research Office (1973) Medicinal plants of Daxing'an Mountains. J Shenyang Pharm Univ 1:35–77
Deng X (2010) Macrofungi In Daxinganling Mts-economic fungi. Northeast Forestry University Press, Harbin
Fu PY (2004) Herbs of Northeast China. Science Press
Fu YY, Zhao JJ, Zhang HY, He HS, Guo XY (2016) Spatiotemporal variation of vegetation phenology in the Daxing’an Mountains stratified by eco-geographical regions. Chin J Appl Ecol 27(9):2797–2806
Gu Y, Han SJ, Zhang JH, Chen ZJ, Wang WJ, Feng Y, Jiang YG, Geng SC (2020) Temperature-dominated driving mechanisms of the plant diversity in temperate forests. Northeast China For 11(2):227. https://doi.org/10.3390/f11020227
Guan WB, Chen T, Dong YK, Zhou YL (1997) Vegetation diversity in northeastern China I. Diversity of vertical vegetation composition in cold temperate coniferous forest region. Chin J Appl Ecol 8(5):465–470. https://doi.org/10.13287/j.1001
Halme P, Holec J, Clausen JH (2017) The history and future of fungi as biodiversity surrogates in forests. Fungal Ecol 27:193–201. https://doi.org/10.1016/j.funeco.(2016).10.005
Han J, Wen RY, Chi ZY (2004) A brief discussion on forest vegetation distribution in Da, XiaoXing’an mountains. Inner Mongolia Sci Technol Econ 16:111–113
Huang JB (1959) A brief introduction of the natural geographical landscape of Arshan area in the south of Daxinganling Mountains. For Sci 6:446–451
Huang NL (1998) Primary color map of Chinese macrofungi. China Agriculture Press, Beijing
Kirk PM, Cannon PF, Minter DW, Stalper JA, Andrianova TV, Aptroot A, Benny GL (2008) Dictionary of the Fungi. CAB international-Europe UK, Wallingford
Li WH (1982) Study on the community structure of larch forest in the south slope of Xiaoxing’an mountains. J Beijing For Univ 3:42–51
Li XP, Wang LJ, Wei L, Xu TJ (2011) A Study on macrofungi resources in Huzhong Nature Reserve. For Sci Technol Inf 43(03):12–13
Liu SE (1955) Flora of woody plants in northeast China: Science Press
Liu HF (1990) 30 years’ change of forest resources in Daxing’an mountains forests. Forest Resour Manag 1:20–26
Liu XD (2004) Color atlas of wild macrofungi in China. China Forestry Publishing House, Beijing
Liu LX (2012) Study on the ecological functional service value of plant diversity of forest ecosystem in Xiaoxinganling. Northeast Forestry University
Liu HH (2013) Macrofungi diversity of Heilongjiang Nanwenhe national nature Reserve. Jilin Argricultural University, Changchun
Ma F (2013) Macrofungi diversity of Mudanfeng national nature reserve of Heilongjiang province. Jilin Agricultural University, Changchun
Ma KP, Huang JH, Yu SL, Chen LZ (1995) Study on Richness, Evenness and Species diversity index of plant community diversity in Dongling Mountain. Beijing Acta Ecol Sin 15(3):268–277
Ma JY, Feng XQ, Guo YC (2000) Study on species diversity of primitive spruce forest in Daxinganling Mountains. For Investig Des 1:54–55
Mao XL (2000) The macrofungi In China. Science and Technology Publishing House of Henan Province, Zhengzhou
Mao ZH, Zhu JJ (2006) Effects of disturbances on species composition and diversity of plant communities. Acta Ecol Sin 26(8):2695–2701
McKinney ML (2006) Urbanization as a major cause of biotic homogenization. Biol Cons 127(3):247–260. https://doi.org/10.1016/j.biocon.(2005).09.005
Mysological Society of China (2016) Nanhua Declaration on the Wild Mushrooms Conservation. Accessed (2016)
Nature Museum Editorial Board (2014) Mushroom. Chemical Industrial Press, Beijing
Nie SQ (1980) Medicinal plants of northeastern forests. Nat Resour Res 2:30–35
Pandolfi JM, Staples TL, Kiessling W (2020) Increased extinction in the emergence of novel ecological communities. Science 370(6513):220–222. https://doi.org/10.1126/science.abb3996
Qian XY (2014) Analysis of forestry resources and non-wood fiber supply in China. China Pulp Paper Ind 9:23–27
Ren LJ, Li GT, Sun XJ, Yu SP, Zheng XW, Yue YJ (2013) Research on community structure and species diversity of Larix gmelinii community in Inner Mongolia Daxing’anling Mountains. Forest Resour Manag 3:83–118. https://doi.org/10.13466/j.cnki.lyzygl.(2013).03.023
Shao J, Wang Z, Zhao YH (1960) Logging and reforestation in Northeast China. For Sci 3:200–207
Shao LP, Xiang CT (1997) Forest mushroom of China. Northeast Forestry University Press, Harbin
Shao LP, Xiang CT (2017) China Forest Mushroom. Northeast Forestry University Press, Harbin
Song GL, Yang GT (2001) Comparison of species diversity between Larix gmelini pure forest and Larix gmelini–Betula platyphylla mixed forest in Daxing’an Mountains. J For Res 12(2):136–138
Song P, Liu Z (1995) Research on forest plant resources and their development and utilization in Daxing’an mountains. Inner Mongolia For Investig Des 4(27):24–27
Sun JX, Du HJ, Zhang JY, Xiang CT, Wang WJ (2020) Survey of fungal resources in Huzhong National Nature Reserve and adjacent areas of Daxing’an Mountains. Edible Fungi 42(2):20–27
Sun MX (2006) Forest plants of Daxinganling. Northeast Forestry University Press
Tobner CM, Paquette A, Gravel D, Reich PB, Williams LJ, Messier C (2016) Functional identity is the main driver of diversity effects in young tree communities. Ecol Lett 19(6):638–647. https://doi.org/10.1111/ele.12600
Wang WJ (2019) Harbin urban forest characteristics and ecological service functions. Science Press, Beijing, China
Wang ZS, Zheng FK, Shi C, Lv ZZ (1959) Preliminary investigation of pinus bungeana plantation in Caohekou Forest Region. For Sci 3:172–181
Wang XG, Zhang TX, Wang B (2012) Eatible mushroon industry development in NE China and future prospect. North Hortic 9:203–205
Wang Q, Zhang D, Zhou W, He XY, Wang WJ (2020a) Urbanization led to a decline in glomalin-soil-carbon sequestration and responsible factors examination in Changchun, Northeastern China. Urban For Urban Green 48:126506. https://doi.org/10.1016/j.ufug.(2019).126506
Wang YY, Yu JH, Xiao L, Zhong ZL, Wang Q, Wang WJ (2020b) Dominant species abundance, vertical structure and plant diversity response to nature forest protection in Northeastern China: conservation effects and implications. Forests 11(3):295. https://doi.org/10.3390/f11030295
Wei SC, Wang QY, Liu XZ, Zhang YL, Fan F (1994) Differentiation of vegetation on the east and west sides of the middle part of the Daxingan Mountains. Acta Pratacul Sin 3(2):12–19
Wu F, Zhou LW, Yang ZL, Bau T, Li T, Dai YC (2019) Resource diversity of Chinese macrofungi: edible, medicinal and poisonous species. Fungal Divers 98:1–76
Xiang CT (2005) Wild eatable mushroom. China Forestry Publishing House, Beijing
Xie ZG (2017) Research on original forest flora and floristic geographic elements in the North Greater Hinggan mountains of Inner Mongolia. For Resour Manag 2:88–92
Xu HC, Li Z, Qiu Y (1997) Fire disturbance history in virgin forest in northern region of Daxinganling Mountains. Acta Ecol Sin 17(4):337–343
Xu LN, Jin GZ (2012) Species composition and community structure of a typical mixed broadleaved-Korean pine (Pinus koraiensis) forest plot in Liangshui Nature Reserve. Northeast China Biodivers Sci 20(4):470–481
Yang Y (2018) Discussion on the present situation and measures of forest pest control. South China Agric 12(33):66–67. https://doi.org/10.19415/j.cnki.1673-890x.(2018).33.035
Yang Y (2019) Study on the effects of fire on forest carbon stocks, plant composition diversity and community structure in Daxingan Mountains. Northeast Forestry University
Yang HS, Wang CB, Qi XL, Wang CP (2017a) Floristic study of rosaceae in the Xiaoxing’an mountains. J Anhui Agric Sci 45(15):4–5
Yang LB, Sui X, Zhu DG, Cui FX, Li JB, Song RQ (2017b) Study on fungal communities characteristics of different Larix gmelini forest typesin cold temperate zone. J Central South Univ For Technol 37(12):76–84
Yang YB, Lv HL, Fu Y, He XY, Wang WJ (2019) Associations between road density, urban forest landscapes, and structural-taxonomic attributes in Northeastern China: decoupling and Implications. Forests 10(1):58
Yu T, Wang W, Zhao BS, Lian J (2005) Economic mcrofungi in Daxinganling Mts in Inner monglica. Northeast Forestry University Press, Harbin
Zhang L, Yuan XY, Zhang DL (2007) The species diversity of the floras of Daxing’an Mountain, Xiaoxing’an Mountain and Ecotone. Bull Bot Res 27(3):356–360
Zhang P (2017) Diversity of Macrofungi in the Greater and Lesser Khinggan Mountains. Jilin Agricultural University, Changchun
Zhang RZ (1965) Pinus camphorae planted on the arid sunny slopes of Daxing ’anling has a preliminary effect. Pract For Technol 13:11
Zhao HX, Wang YH, Li JQ, He XL (1987) Age structure, horizontal pattern and management of Natural Larch Forest in Tahe Forestry Bureau. J Northeast For Univ 15:60–64
Zhou YL (1991) Vegetation of Daxinganling in China. Science Press
Zhou YL (1994) Vegetation of Xiaoxinganling in China. Science Press
Zhou YL (1997) Vegetation geography of Northeastern China. Science Press, Beijing
Zhou YL, Zhao G (1964) The type, distribution and succession of natural secondary forest in Changbai Mountain forest area-Xiaoxinganling Mountains. J Northeast For Inst 3:33–44
Zhou RC, Zhou YL (1985) Plant resources of Heilongjiang province. Territory Nat Resour Study 4:19–28
Zhou YL, Li JW (1964) The characteristics and distribution of the main vegetation types in the eastern mountainous areas of Northeast China. Chin J Plant Ecol 2(2):190–205
Zhou YL, Wu HQ, Chen T, Zheng YR (1989) Accelerating the recovery of forest in the Burned Area in Daxing’anling according to the synecological characteristics. J Northeast For Univ 17(8):2–9
Zhou YL, Ni HW, Zhou RC (1998) Characteristics of forest plant diversity in Daxing'an Mountains. Territory Nat Resour Study 3(16):66–68
Acknowledgements
We would like to thank Hongju Du, Jianyu Zhang and many other students for their dedication to our fieldwork. Acknowledgements are also due to Prof. Cunti Xiang (retired from Northeast Forestry University) for his kind help on macrofungi recognition.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Project funding: This work was supported by the National Natural Science Foundation of China (Grant No. 41730641); Project from Ministry of Science and Technology of China (Basic Research project: 2014FY110600 and 13-5 Project: 2016YFA0600802).
The online version is available at http://www.springerlink.com.
Corresponding editor: Yu Lei.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, Y., Wen, H., Wang, K. et al. Forest plant and macrofungal differences in the Greater and Lesser Khingan Mountains in Northeast China: A regional-historical comparison and its implications. J. For. Res. 33, 623–641 (2022). https://doi.org/10.1007/s11676-021-01313-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11676-021-01313-z