Abstract
Agarwood is the resinous heartwood of Aquilaria species. However, low yields and high costs of existing stimulation methods have led to the need for new techniques to produce agarwood rapidly and effectively. We developed a biological agarwood-inducing technique (Agar-Bit) that produces high yields and quality within 6 months. To better understand agarwood formation by Agar-Bit, dynamic gene expressions of key synthetases pathways of sesquiterpenes and chalcone- related enzymes at different times were determined after both Agar-Bit and the traditional burning chisel drilling (BCD) stimulation on Aquilaria sinensis trees. The qRT-PCR results show that some characteristic synthase genes were expressed at greatly different levels and times compared with the controls. For the Agar-Bit technology, main changes were after the 3rd or 5th month, while BCD expression clearly changed at the 5th month. Essential oils and total chromone contents were simultaneously determined. In the Agar-Bit group, both were higher and similar to natural levels. The Agar-Bit methodology is a new option for producing agarwood as demonstrated by genetic and chemical aspects. The differences in gene expression within 6 months for both groups indicates that the mechanisms of the two methods are different. These findings provide information on genetic variation during the process of agarwood formation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Agarwood, resinous trunks or branches from Aquilaria trees, is widely used in perfume manufacturing, the production of incense and in traditional Chinese medicine. However, agarwood is only harvested from wounded trees. In nature, agarwood is formed by insect feeding, lightning strikes or storm damage and result in low production, keeping prices high. There was previously a problem for natural Aquilaria because of uncontrolled slashing for economic benefits, leading to all Aquilaria species being listed in Appendix II of the Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES 2004). It is still important to find new methodologies to improve production and quality.
Existing methods for producing agarwood are time-consuming and expensive, and result in low yields. These methods include partial trunk pruning, wounding the trunk by hammering of nails into the wood, burning, chiseling, and drilling, and fungal inoculation. Natural processes often require three to 4 years to form agarwood of reasonable quality, and 10 years or more to produce high-quality agarwood. Newer methods, including cultivated agarwood kits (Blanchette and Van Beek 2009) and a whole-tree agarwood- inducing technique (Liu et al. 2013) are being evaluated and require improvement before practical promotion, especially in regards to mechanism and quality. Our team has developed a method combining biochemical and physical treatments to induce agarwood production in Aquilaria sinensis (Lour.) Gilg within 6 months (Lin et al. 2015; Wu et al. 2017).
It is important to understand the biosynthesis and regulation of sesquiterpenes and chromones in Aquilaria spp. in order to determine the mechanism of agarwood formation. The biosynthesis of sesquiterpenes is via the mevalonic acid and 1-deoxy-D-xylulose-5-phosphate (Gardner and Hampton 1999; Rohmer 1999) pathways in which sesquiterpene synthases are enzymes in the final step of forming sesquiterpenes. It has been reported that both resin and terpenoids in other plants are produced in response to biotic and abiotic stresses (Lewinsohn et al. 1991). However little is known about the biosynthesis of fragrant compounds such as sesquiterpenes. Uncovering the mechanism of agarwood formation requires studying the stress response by Aquilaria trees, especially changes in gene expression levels following stimulation. Applications of RNA-sequencing and digital gene expression profiling provide adequate molecular information concerning biosynthesis pathways. A transcriptome library has been established between healthy and wounded A. sinensis over time and relevant genes involving signal transduction selected. With this molecular foundation, 25 relevant A. sinensis genes have been cloned which play vital roles in the synthesis of key enzymes in the sesquiterpene and flavonoid pathways.
Sesquiterpene and 2-(2-phenylethyl) chromones from essential oils are the two main chemicals in agarwood. Essential oils consist of characteristic sesquiterpenes and aromatic chemicals which have sedative and analgesic effects (Okugawa et al. 1996, 2000). Chromones in agarwood have recently been found to be important for identification purposes and for their pharmacological effects. Analysis of 2-(2-phenylethyl) chromones is promising to distinguish cultivated from wild harvested agarwood, especially the highly oxidized 5,6,7,8-tetrahydro-2-(2-phenylethyl) chromones (Espinoza et al. 2014). Some new chromones in agarwood show antitumor immunity (Suzuki et al. 2017), inhibition of innate and adaptive immunity (Guo et al. 2017) and anti-inflammatory properties (Zhu et al. 2016; Huo et al. 2017). More chromones have been isolated from agarwood in recent years (Liao et al. 2016, 2017; Xiang et al. 2017; Yang et al. 2017). The determination of chromones is more useful than other aromatic compounds for agarwood identification.
The exact process of agarwood formation remains unclear. Many chemicals have been reported as autoinducers that stimulate agarwood formation. Some fungi appear to induce the molecular mechanism of agarwood formation (Chen et al. 2017; Chhipa and Kaushik 2017; Sen et al. 2017). More research on satisfactory models with high agarwood production is required to uncover the mechanism of agarwood formation. Our method can be a good option to stimulate production because of high yields and quality of agarwood. Since it takes time for agarwood to form, a dynamic study at the molecular level is needed. Some fungal succession was detected within 12 months using quantitative reverse transcription (qRT)-PCR and revealed the interaction of fungi and agarwood formation (Mohamed et al. 2014).
The aim of this study was to determine the mechanism of agarwood formation using our Agar-Bit method, thereby providing information on chemicals and genes. Gene expression for biosynthesis enzymes of sesquiterpenes and flavonoids were studied over time, and changes detected using qRT-PCR within six months. The components of essential oils and total chromones were determined for agarwood quality.
Materials and methods
Plant materials and chemicals
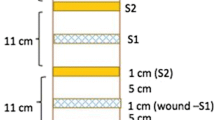
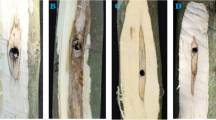
Five-year-old A. sinensis saplings cultivated in the wild in Guangzhou, Guangdong Province were used for bio-physical and burning-chiseling-drilling (BCD) treatments. Details of the Agar-Bit method are found in Lin et al. (2015) and Wu et al. (2017). The BCD treatment was carried out using a hot chisel as this is the traditional method to produce stable quality agarwood for Chinese Pharmacopeia (2015). Samples of heartwood were collected with a chisel at different times (at the beginning, and every month for 6 months after injury. Genetic samples were taken 1.5 − 2.0 cm in the xylem and immediately stored in liquid nitrogen. Each month discolored parts of each sapling were collected to compare with natural agarwood for quality. Wild cultivated A. sinensis and natural agarwood were purchased from the Guangdong Qingping Market (In Liwan district, Guangzhou, Guangdong province) and identified by Prof. Chaomei Pan of the Chinese Medicine College, Guangzhou University of Chinese Medicine, Guangzhou. Herbal materials were deposited in the Experimental Management Center in the College. Plant materials of the Agar-Bit and BCD methods are shown in Fig. 1.
HPLC (High Performance Liquid Chromatography) grade methanol, ethanol and other chemicals were purchased from Merck & Co., Inc. New Jersey, USA). Aquilarone E as the chromone standard was prepared in our laboratory with a minimum of 98% purity.
Gene expression
Information on determining gene expression is given in Wu et al. (2017). Total RNA was extracted from all samples using the optimized method, and the quantity and quality were determined by a microplate spectrophotometer. A 5 μL RNA quantity for each sample was used in the 20 μL reverse-transcription reaction system, with PrimeScript™ RT reagent Kit and gDNA Eraser following the manufacturer’s instruction. Primers were designed with Primer 5.0 software from genomic BLAST databases. Sequences of histone primers were obtained from Kumeta and Ito (2010). Control means wood samples at 0 h (sapwood) in both Agar-Bit and BCD groups. Real-time PCR reaction was performed using SYBR® Premix Ex Taq™ qRT-PCR for the evaluation of all genes in biological duplicate. Each PCR was repeated three times. The specificity of the qRT-PCR reaction was determined by melt curve analysis of the amplified products with heating from 55 °C to 65 °C in 0.5 °C steps. Raw expression data were analyzed by BioRad CFX Manager Software version 2.0. All amplification plots were analyzed with a threshold fluorescence of 0.1 to obtain cycle threshold (Ct) values. The expression levels were evaluated by the 2−∆∆Ct method and analyzed using Excel 2010.
Essential oil content
Samples were ground to powder, passed through a 20-mesh sieve and moisture content determined by the toluene method according to China Pharmacopeia (2015). Essential oils were extracted with Et2O according to Wu et al. (2017). Samples (10 g) were soaked overnight in diethyl ether and filtered twice at 25 °C. The filtered liquor was collected and volatilized at room temperature. Methylene dichloride was added as the solvent and left standing for 48 h. The mixture was filtered, volatilized and weighed. The remaining liquor was dried over hydrous sodium sulfate and stored at − 20 °C until analysis. Essential oil contents were calculated with the weight of oil extraction in crude material (w/w%). Control means wood samples at 0 h (sapwood) in both Agar-Bit and BCD groups.
Results and discussion
Gene expression
RNA integrity was assessed by the sharpness of ribosomal RNA (rRNA) bands visualized on a denaturing 1.2% agarose gel. For all samples, well-resolved 28 S and 18 S rRNA bands were observed with no visible signs of degradation (Fig. 2). There were gDNA bands observed so the elimination of gDNA was a precondition for further molecular analysis. A260/A280 ratios ranged from 1.90 to 1.99 (average = 1.94, coefficient of variation (CV) = 1%), indicating low or no protein contamination. The yields of total RNA were 254–312 μg/g FW (fresh weight). For the whole set of samples, the average yield was 287 μg RNA/g FW with a CV of 21%. The A260/A230 ratio exceeded 2.0 for all samples (average = 2.3, CV = 6.1%). Overall, these data demonstrate that the extraction protocol was efficient in yielding high quality, integrity and quantity of total RNA from A. sinensis.
A qRT-PCR method measured the transcript levels for genes and gene expression throughout the time course was monitored with histone as the reference gene. Melting curve analysis confirmed that the primers amplified a single product. A fivefold dilution series of one of the samples was used to prepare a standard curve from which primer efficiency was calculated using the formula E = 10−1/SLOPE. Proper Tm (melting temperature) was selected for different genes as described previously (Wu et al. 2017).
After the establishment of optimal qRT-PCR conditions, all samples from the different groups and times were analyzed for gene expression with values at 0 h used as controls (Figs. 3, 4). The enzymes 1-deoxy-D-xylulose-5-phosphate synthase 1 (DXS1), 3-hydroxy-3-methylglutaryl-coenzyme a reductase (HMGR), and farnesyl diphosphate synthase (FPS) are in the upper-stream of the sesquiterpene pathway. For the Agar-Bit group, genes for these three enzymes were down-regulated after stimulation and their expressions reached a maxima at 1 month for AsDXS1 and at 3 months for the others. By 4 months, expressions of all three had returned to minima. The up-regulation ranges of the three genes were similar. For the BCD group, the three genes showed highest expressions at 5 months, especially for FPS which reached 100-fold above the control. All three genes were sharply down-regulated at 6 months.
Sesquiterpene synthase 1 (AsSS1) and terpene synthase (TPS) are strongly specific in the sesquiterpene pathway. The results demonstrate that AsSS1 expression clearly decreased after stimulation by the Agar-Bit method, but reached a maximum at 3 months. For the BCD group, AsSS1 was highly expressed at 5 months, with a 1000-fold increase over the control. AsTPS expression in the BCD group differed from that in the Agar-Bit group, with the highest expression at 6 months, followed by 5 months. After BCD stimulation, AsTPS at 5 months was almost 1100 times the original value; however, the rate of increase was nonlinear.
Chalcone synthase 1 (CHS1) and chalcone isomerase 1 (CHI1) are the key enzymes in the flavonoid pathway. Because unique chromones in agarwood play important roles in its chemical composition, and the main structure of chromones is similar to that of flavonoids, regulation of the two genes can provide information on the chromone biosynthesis pathway. The CHI1 showed maximum expression at 3 months in the Agar-Bit and at 5 months in BCD group. CHS1 expression was down-regulated in the Agar-Bit but sharply up-regulated at 5 months in BCD group to 600-fold over the control.
The TPS family in plants encodes enzymes that use similar substrates and gives similar products but has clearly diverged in different lineages (Jorg et al. 1998). Wu et al. (2013) cloned sesquiterpene synthase from A. sinensis, and an analysis based on the literature and orthologous sequences from the NCBI revealed that its coding protein shared 68% similarity with putative (-)-germacrene-D synthase of Vitis vinifera L., and their consistency was 50%. Without transmembrane domain, As-SesTPS protein was located in the cytoplasm and expressed only in the agarwood formation area. A phylogenetic analysis suggested that the AsHMGR protein sequence had high similarity to those of Arabidopsis thaliana (L.) Heynh., Arabidopsis lyrata subsp. lyrata and Brassica juncea (L.) Czern. et Coss. and that AsHMGR transcription could be induced by methyl jasmonate (MJ) (Liu et al. 2014). FPS is a key rate-limiting enzyme in the sesquiterpene metabolic pathway. Three full-length cDNAs of ASS1-3 were cloned and expressed in E. coli, and enzyme assays revealed that they resulted in active enzymes with the major product being δ-guaiene. A MJ- induction experiment showed that ASS (1–3) expression was significantly induced by MJ and the production of sesquiterpenes was elevated accordingly. Some transcription factors and protein kinases, such as V-myb avian myeloblastosis viral oncogene homolog 4, WRKY4, and mitogen-activated protein kinase 2, may be positive regulators of ASS (Xu et al. 2014). DXS is the first rate-limiting enzyme for sesquiterpene synthesis in the MEP (2C-methyl-D-erythritol 4-phosphate) pathway. Two kinds of cDNAs encoding DXS1 from A. sinensis were cloned. AsDXS1 was significantly stimulated by mechanical, chemical and hydrogen peroxide treatments and oscillated in response to MJ treatment (Xu et al. 2014). These genes are involved in upstream and downstream terpene pathways. CHI1 and CHS1 are critical in the flavonoid pathway. The similar basic structures of flavonoids and chromones indicate some biosynthesis information. AsCHS1 transcripts were most significantly induced by salt stress, enhanced by gibberellins, MJ or salicylic acid treatment, whereas Type III polyketide synthase genes displayed low transcript levels at the early stage under abscisic acid treatment (Wang et al. 2017). The CHS1 and CHS2 genes were expressed differently with formic acid stimulation combined with Fusarium spp. A2 inoculation within a year (Chen et al. 2017). These genes found in A. sinensis will help to better understand the inner mechanisms of agarwood formation at the gene level.
These genes were expressed differently between the two induction groups. For Agar-Bit, DXS1, HMGR, FPS and CHS1 were down-regulated; others were initially down-regulated and then mainly up-regulated after 3 months. For the BCD group, these genes were mostly up-regulated in a similar pattern and were highly expressed by 5 months. In addition, these genes were expressed more highly than in the Agar-Bit group, with ASS1 and TPS by almost 1000 times. It is apparent that the inner mechanisms of the induction methods clearly differed.
Within 6 months, the Agar-Bit triggered the agarwood formation process at a low level, but the BCD treatment sharply initiated the process by 5 months. Further study should focus on this distinction to determine the actual and systemic mechanism of agarwood formation. Agarwood is mainly formed by 6 months and this dynamic gene study over time has provided more information on the formation process. Compared with the results of a previous study on the expression of these genes at an early stage (within 48 h) (Wu et al. 2017), dynamic gene expressions within 6 months were mainly down-regulated, except for ASS1, TPS and CHI1 according to Agar-Bit. For other inducing methods, gene expression varied during 12 months, with CHS1 and CHS2 still up-regulated at 12 and 10 months, respectively (Mohamed et al. 2014). Various triggers such as fungi, wounding, insect attack, fire and chemicals may activate defense reactions and result in the production of triterpenoids and chromones. However, different triggers may involve different defense reaction patterns over time as well as different gene type and expression intensity, as shown for key biosynthesis enzyme genes in this study after treatment with Agar-Bit and BCD. These preliminary results may explain the different genetic mechanisms of specific compounds in agarwood.
Essential oil content
Average moisture content of all Agar-Bit and BCD groups were 4.9% and 6.8%, respectively. Yields of essential oils after Et2O (diethyl ether) extraction are shown in Fig. 5. Agar-Bit group yields were higher during the first four to 6 months, and by the end of 6 months were almost fivefold of those of the BCD group. Wu et al. (2017) determined the essential oil content of natural agarwood (2.8%) over a control (0.2%). Common essential oil extraction methods for agarwood are steam distillation and the use of solvents. However, since agarwood is expensive, Et2O is a commonly used solvent (Mei et al. 2013), especially for further analysis with GC–MS. We found that Et2O the main sesquiterpenes and chromones of agarwood could be extracted and thus the essential oil could be a simple and stable parameter for agarwood quality control.
Total chromone analysis
Chromones in agarwood have recently received considerable attention for their importance in identification of quality (Espinoza et al. 2014), pharmacological effects (Zhu et al. 2016; Huo et al. 2017; Suzuki et al. 2017), chemical isolation (Liao et al. 2016, 2017; Xiang et al. 2017; Yang et al. 2017), and their biosynthesis pathways (Wang et al. 2016). The determination of chromones in agarwood is a reasonable option for quality control (Lancaster and Espinoza 2012). The colorimetric method used in this study determined the chromone content and is simple and stable to use for quality control. The total chromone content of the Agar-Bit group was higher during 4–6 months, and after 6 months, levels were almost twice those of the BCD group (Fig. 6). Wu et al. (2017) determined the chromone content of natural agarwood as 19.4% and 2.3% for controls; the content using the Agar-Bit method was close to the natural level.
Conclusions
In this study, dynamic expression of genes for key synthetases in the sesquiterpene pathway and for chalcone-related enzymes that varied over time were determined after both Agar-Bit and burning-chiseling-drilling stimulation on A. sinensis. The dynamic regulation of these gene expressions were analyzed by comparing two treatment groups as well as determining essential oil and chromone contents. Genetic results showed that regulation of gene expressions was still active within 6 months. However, the genes were expressed differently according to the method of induction, time and gene type. The response mechanism may vary according to the inducing method as shown by the maxima for the BCD method being after 5 months but not for Agar-Bit method. In addition, comparisons of essential oils and chromone contents showed that the Agar-Bit method was superior for the production of quality agarwood because these characteristic compounds were higher in agarwood during 6 months than they were for the BCD group. These results show dynamic variations in characteristic compounds and gene expression of key synthetases. Because systemic information of molecular mechanisms remains limited, revealing the elaborate biosynthesis pathways of specific chemicals in agarwood will require more combination of related gene information and precise determination of compounds during agarwood formation. In further studies, application of more chromone-related synthetase genes and more precise chemical detection based on Aquilaria models may reveal the complex process of agarwood formation. The Agar-Bit method is a novel option for producing agarwood as shown by genetic and chemical aspects.
Change history
05 July 2019
The article “Dynamic analysis of gene expression and determination of chemicals in agarwood in Aquilaria sinensis”, written by Zeqing Wu, Wanzhen Liu, Jing Li, Liangwen Yu and Li Lin was originally published electronically on the publisher’s internet portal (currently SpringerLink) on 10th May 2019 without open access.
References
Blanchette RA, Van Beek HH (2009) Cultivated agarwood. U.S. Patent No. 7638145B2. Minnesota: University of Minnesota
Chen XD, Zhu XL, Feng MR, Zhong ZJ, Zhou X, Chen XY, Ye W, Zhang WM, Gao XX (2017) Relationship between expression of chalcone synthase genes and chromones in artificial agarwood induced by formic acid stimulation combined with Fusarium spp. A2 inoculation. Molecules 22 (5):686
Chhipa H, Kaushik N (2017) Fungal and bacterial diversity isolated from Aquilaria malaccensis trees and soil induces agarospirol formation within 3 months after artificial infection. Front Microbiol 7:128
CITES (2004) Amendments to appendices I and II of CITES. In: Proceedings of thirteenth meeting of the conference of the Parties, Bangkok, Thailand, 2 October
Espinoza EO, Lancaster CA, Kreitals NM, Hata M, Cody RB, Blanchette RA (2014) Distinguishing wild from cultivated agarwood (Aquilaria spp.) using direct analysis in real time and time of flight mass spectrometry. Rapid Communications in Mass Spectrometry 28 (3):281 − 289
Gardner RG, Hampton RY (1999) A highly conserved signal controls degradation of 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase in eukaryotes. J Biol Chem 274(44):31671–31678
Guo R, Zhao YF, Li J, Gu YF, Huo HX, Li SS, Song YL, Zhu ZX, Tu PF (2017) GYF-21, an epoxide 2-(2-Phenethyl)-chromone derivative, suppresses innate and adaptive immunity via inhibiting STAT1/3 and NF-kB signaling pathways. Front Pharmacol 8:281
Huo HX, Gu YF, Sun H, Zhang YF, Liu WJ, Zhu ZX, Shi SP, Song YL, Jin HW, Zhao YF, Tu PF, Li J (2017) Anti-inflammatory 2-(2-phenylethyl) chromone derivatives from Chinese agarwood. Fitoterapia 118:49–55
Jorg B, Gilbert MG, Rodney C (1998) Plant terpenoid synthases: molecular biology and phylogenetic analysis. Proc Natl Academy Sci U S A 95(8):4126–4133
Kumeta Y, Ito M (2010) Characterization of delta-guaiene synthases from cultured cells of Aquilaria, responsible for the formation of the sesquiterpenes in agarwood. Plant Phys 154(4):1998–2007
Lancaster C, Espinoza E (2012) Evaluating agarwood products for 2-(2-phenylethyl) chromones using direct analysis in real time time-of-flight mass spectrometry. Rapid Commun Mass Spectrom 26(23):2649–2656
Lewinsohn E, Gijzen M, Croteau R (1991) Defense mechanisms of conifers: differences in constitutive and wound − induced monoterpene biosynthesis among species. Plant Physiol 96(1):44–49
Liao G, Mei WL, Dong WH, Li W, Wang P, Kong FD, Gai CJ, Song XQ, Dai HF (2016) 2-(2-Phenylethyl) chromone derivatives in artificial agarwood from Aquilaria sinensis. Fitoterapia 110:38–43
Liao G, Mei WL, Kong FD, Li W, Yuan JZ, Dai HF (2017) 5,6,7,8-tetrahydro-2-(2-phenylethyl) chromones from artificial agarwood of Aquilaria sinensis and their inhibitory activity against acetylcholinesterase. Phytochemistry 139:98–108
Lin L, Wu ZQ, Cai DY, Shuai O, Li MC (2015) A combination and a method for holistic agarwood production in Aquilaria sinensis. CN 201510259894.0
Liu YY, Chen HQ, Yang Y, Zhang Z, Wei JH, Meng H, Chen WP, Feng JD, Gan BC, Chen XY, Gao ZH, Huang JQ, Chen B, Chen HJ (2013) Whole-tree agarwood-inducing technique: an efficient novel technique for producing high-quality agarwood in cultivated Aquilaria sinensis trees. Molecules 18(3):3086–3106
Liu AG, Juvik JA, Jeffery EH, Berman-Booty LD, Clinton SK, ErdmanJr JW (2014) Enhancement of broccoli indole glucosinolates by methyl jasmonate treatment and effects on prostate carcinogenesis. J Med Food 17(11):1177–1182
Mei WL, Yang DL, Wang H, Yang JL, Zeng YB, Guo B, Dong W, Li W, Dai H (2013) Characterization and determination of 2-(2-phenylethyl) chromones in agarwood by GC-MS. Molecules 18(10):12324–12345
Mohamed R, Jong PL, Irdayu IN (2014) Succession patterns of fungi associated to wound-induced agarwood in wild Aquilaria malaccensis revealed from quantitative PCR assay. World J Microbiol Biotechnol 30(9):2427–2436
Okugawa H, Ueda R, Matsumoto K, Kawanishi K, Kato A (1996) Effect of jinkoh-eremol and agarospirol from agarwood on the central nervous system in mice. Med Plant Nat Prod Res 62(1):2–6
Okugawa H, Ueda R, Matsumoto K, Kawanishi K, Kato K (2000) Effects of sesquiterpenoids from “Oriental incenses” on acetic acid-induced writhing and D2 and 5 − HT2A receptors in rat brain. Phytomedicine 7(5):417–422
Rohmer M (1999) The discovery of a mevalonate-independent pathway for isoprenoid biosynthesis in bacteria, algae and higher plants. Nat Prod Rep 16(5):565–574
Sen S, Dehingia M, Talukdar NC, Khan M (2017) Chemometric analysis reveals links in the formation of fragrant bio-molecules during agarwood (Aquilaria malaccensis) and fungal interactions. Sci Rep 7:44406
Suzuki A, Miyake K, Saito Y, Rasyid FA, Tokuda H, Takeuchi M, Suzuki N, Ichiishi E, Fujie T, Goto M, Sasaki Y, Nakagawa-Goto K (2017) Phenylethylchromones with in vitro antitumor promoting activity from Aquilaria filaria. Planta Med 83(3–04):300–305
Wang XH, Gao BW, Liu X, Dong XJ, Zhang ZX, Fan HY, Zhang L, Wang J, Shi SP, Tu PF (2016) Salinity stress induces the production of 2-(2-phenylethyl) chromones and regulates novel classes of responsive genes involved in signal transduction in Aquilaria sinensis calli. BMC Plant Biol 16(1):119
Wang XH, Zhang ZX, Dong XJ, Feng YY, Liu X, Gao BW, Wang JL, Zhang L, Wang J, Shi SP, Tu PF (2017) Identification and functional characterization of three type III polyketide synthases from Aquilaria sinensis calli. Biochem Biophys Res Commun 486(4):1040–1047
Wu HQ, Wang L, He X, Bai L, Gao XX, Yan HJ, Zhang WM (2013) Molecular cloning of sesquiterpene synthase gene As-Ses TPS from Aquilaria sinensis and its bioinformatics and expression analysis. Chin Tradit Herb Drugs 45:94–101
Wu ZQ, Liu S, Li J, Li MC, Du HF, Qi LK, Lin L (2017) Analysis of gene expressions and quality of agarwood by Agar-Bit in Aquilaria sinensis (Lour.) Gilg. J Trop Forest Sci 29(3):380–388
Xiang P, Mei WL, Chen HQ, Li W, Chen ZB, Dai HF (2017) Four new bi-phenylethylchromones from artificial agarwood. Fitoterapia 120:61–66
Xu YH, Liu J, Liang L, Yang X, Zhang Z, Gao ZH, Sui C, Wei JH (2014) Molecular cloning and characterization of three cDNAs encoding 1-deoxy-d-xylulose-5-phosphate synthase in Aquilaria sinensis (Lour.) Gilg. Plant Physiol Biochem 82:133–141
Yang Y, Mei WL, Kong FD, Chen HQ, Li W, Chen ZB, Dai HF (2017) Four new bi-2-(2-phenylethyl) chromone derivatives of agarwood from Aquilaria crassna. Fitoterapia 119:20–25
Zhu ZX, Gu YF, Zhao YF, Song YL, Li Jun TuPF (2016) GYF-17, a chloride substituted 2-(2-phenethyl)-chromone, suppresses LPS-induced inflammatory mediator production in RAW264.7 cells by inhibiting STAT1/3 and ERK1/2 signaling pathways. Int Immunopharmacol 35:185–192
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Project funding: The work was supported by Grant Number NDRC2011-51 from the National Development and Reform Commission (NDRC), the Office of New High-Tech Industrial Development. This work was also supported by Grant Number 18A36002 from Key project of institutions of colleges and universities in Henan province, Henan Education Department.
The online version is available at http://www.springerlink.com.
Corresponding editor: Tao Xu.
The original version of this article was revised due to a retrospective Open Access order.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, duplication, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
About this article
Cite this article
Wu, Z., Liu, W., Li, J. et al. Dynamic analysis of gene expression and determination of chemicals in agarwood in Aquilaria sinensis. J. For. Res. 31, 1833–1841 (2020). https://doi.org/10.1007/s11676-019-00970-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11676-019-00970-5