Abstract
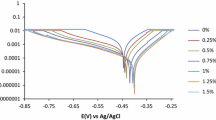
The corrosion inhibition of low-carbon steel in 1 M H2SO4 and HCl solutions by the admixture of Salvia officinalis and Lavandula officinalis essential oil extracts was studied through potentiodynamic polarization analysis, coupon measurement and optical microscopy. The carbon steel undergoes severe surface deterioration in H2SO4, while the morphology of the steel from HCl showed selective deterioration with numerous corrosion pits in the absence of the oil extracts. The extracts performed effectively in the acid media with optimal inhibition efficiency of 86.92 and 96.90% in H2SO4, and 84.68 and 97.59% in HCl from potentiodynamic polarization and coupon analysis. The oil extract displayed anodic inhibition properties in H2SO4 due to surface coverage of the steel and cathodic inhibition in HCl due to selective precipitation of extract molecules. Thermodynamic calculations show the extracts adsorbed onto the steel surface, effectively suppressing the corrosion reactions through chemisorption mechanism according to the Langmuir, Frumkin and Freundlich adsorption isotherms.









Similar content being viewed by others
References
D. Dwivedi, K. Lepkov, T. Becker, Carbon steel corrosion: A review of key surface properties and characterization methods. RSC. Adv. 7, 4580–4610 (2017)
Y. Li, P. Zhao, Q. Liang, B. Hou, Berberine as a natural source inhibitor for mild steel in 1 M H2SO4. Appl. Surf. Sci. 252, 1245–1253 (2005)
G. Quartarone, L. Ronchin, A. Vavasori, C. Tortato, L. Bonaldo, Inhibitive action of gramine towards corrosion of mild steel in deaerated 1.0 M hydrochloric acid solutions. Corros. Sci. 64, 82–89 (2012)
H. Ashassi-Sorkhabi, M.R. Majidi, K. Seyyedi, Investigation of inhibition effect of some amino acids against steel corrosion in HCl solution. Appl. Surf. Sci. 225, 176–185 (2004)
M. Özcan, AC impedance measurements of cysteine adsorption at mild steel/sulphuric acid interface as corrosion inhibitor. J. Solid State Electrochem. 12, 1653–1661 (2008)
R.T. Loto, E. Oghenerukewe, Inhibition studies of rosmarinus officinalis on the pitting corrosion resistance 439LL ferritic stainless steel in dilute sulphuric acid. Orient. J. Chem. 32(5), 2813–2832 (2016)
J. Fu, S. Li, L. Cao, Y. Wang, L. Yan, L. Lu, l-Tryptophan as green corrosion inhibitor for low carbon steel in hydrochloric acid solution. J. Mater. Sci. 45, 979–986 (2010)
A. Bouoidina, M. Chaouch, A. Abdellaoui, A. Lahkimi, B. Hammouti, F. El-Hajjaji, M. Taleb, A. Nahle, Essential oil of “Foeniculum vulgare”: antioxidant and corrosion inhibitor on mild steel immersed in hydrochloric medium. Anti-Corros. Meths. Mats. 64(5), 563–572 (2017)
E.E. Hamdani, O. Mokhtari, A. Salhi, N. Chahboun, B. ElMahi, A. Bouyanzer, A. Zarrouk, B. Hammouti, J. Costa, Chemical constituents and corrosion inhibition of mild steel by the essential oil of Thymus algeriensis in 1.0 M hydrochloric acid solution. Der Pharma. Chemica. 7(8), 252–264 (2015)
Y. El Ouadi, A. Bouyanzer, L. Majidi, J. Paolini, J.M. Desjobert, J. Costa, A. Chetouani, B. Hammouti, Salvia officinalis essential oil and the extract as green corrosion inhibitor of mild steel in hydrochloric acid. J. Chem. Pharm. Res. 6(7), 1401–1416 (2014)
E. El ouariachi, A. Bouyanzer, R. Salghi, B. Hammouti, J.-M. Desjobert, J. Costa, J. Paolini, L. Majidi, Inhibition of corrosion of mild steel in 1 M HCl by the essential oil or solvent extracts of ptychotis verticillata. Res. Chem. Intermed. 41(2), 935–946 (2015)
K. Boumhara, M. Tabyaoui, C. Jama, F. Bentiss, Artemisia mesatlantica essential oil as green inhibitor for carbon steel corrosion in 1 M HCl solution: Electrochemical and XPS investigations. J. Ind. Eng. Chem. 29, 146–155 (2015)
N. Lahhit, A. Bouyanzer, J.-M. Desjobert, B. Hammouti, R. Salghi, J. Costa, C. Jama, F. Bentiss, F. Majidi, Fennel (foeniculum vulgare) essential oil as green corrosion inhibitor of carbon steel in hydrochloric acid solution. Port. Electrochim. Acta. 29(2), 127–138 (2011)
B. Zerga, M. Sfaira, Z. Rais, M.E. Touhami, M. Taleb, B. Hammouti, B. Imelouane, A. Elbachiri, Lavender oil as an ecofriendly inhibitor for mild steel in 1 M HCl. Mater. Tech. 97, 297–305 (2009)
F.-A. Arash, M. Noori, The study of corrosion inhibition mechanism of one of the Salvia officinalis extract on carbon steel in H2S and HCl solutions. Anal. Bioanal. Electrochem. 8(2), 145–157 (2016)
S.A. Umoren, U.M. Eduok, M.M. Solomon, A.P. Udoh, Corrosion inhibition by leaves and stem extracts of sida acuta for mild steel in 1 M H2SO4 solutions investigated by chemical and spectroscopic techniques. Arab. J. Chem. 9(1), S209–S224 (2016)
O. Ouachikh, A. Bouyanzer, M. Bouklah, J.-M. Desjobert, J. Costa, B. Hammouti, L. Majidi, Application of essential oil of artemisia herba alba as green corrosion inhibitor for steel in 0.5 M H2SO4. Surf. Rev. Lett. 16(1), 49–54 (2009)
G. Cristofari, M. Znini, L. Majidi, A. Bouyanzer, S.S. Al-Deyab, J. Paolini, B. Hammouti, J. Costa, Chemical composition and anti-corrosive activity of pulicaria mauritanica essential oil against the corrosion of mild steel in 0.5 M H2SO4. Int. J. Electrochem. Sci. 6, 6699–6717 (2011)
R. Salghi, H.D. Ben, O. Benali, S. Jodeh, I. Warad, O. Hamed, E.E. Ebenso, A. Oukacha, S. Tahrouch, B. Hammouti, Study of the corrosion inhibition effect of pistachio essential oils in 0.5 M H2SO4. Int. J. Electrochem. Sci. 10, 8403–8411 (2015)
M. Gobara, B. Zaghloul, A. Baraka, M. Elsayed, M. Zorainy, M.K. Mohamed, H. Elnabarawy, Green corrosion inhibition of mild steel to aqueous sulfuric acid by the extract of Corchorus olitorius stems (Res. Exp, Mats, 2017). https://doi.org/10.1088/2053-1591/aa664a
A. Rodriguez-Torres, M.A. Valladares-Cisneros, J.G. Gonzalez-Rodriguez, Use of Salvia officinalis as green corrosion inhibitor for carbon steel in acidic media. Int. J. Electrochem. Sci. 10(5), 4053–4067 (2015)
M.V. Tomić, V.M. Mićić, R.F. Godec, M.G. Pavlović, D. Vaštag, M.G. Riđošić, M.M. Pavlović, Sage extracts as inhibitors of steel corrosion in 4% HCl. Int. J. Electrochem. Sci. 11, 3339–3350 (2016)
R.T. Loto, Corrosion polarization behaviour and inhibition of S40977 stainless steel in benzosulfonazole/3 M H2SO4 solution. South Afr. J. Chem. Eng. 24, 148–155 (2017)
Corrosion of Iron http://tdwhs.nwasco.k12.or.us/staff/bfroemming/CorrosionIron.html. Accessed 12 June 2018
R.T. Loto, C.A. Loto, A.P.I. Popoola, Electrochemical Studies of the corrosion inhibition effect of 2-amino-5-ethyl-1,3,4-thiadiazole on low carbon steel in dilute sulphuric acid. J. Chem. Soc. Pak. 36(6), 1043–1051 (2014)
S.H. Zaferani, M.R. Shishesaz, Corrosion inhibition of carbon steel in acidic solution by alizarin yellow GG (AYGG). J. Pet. Environ. Biotechnol. 5, 4 (2014). https://doi.org/10.4172/2157-7463.1000188
O.M. Magnussen, Corrosion protection by inhibition, encyclopedia of electrochemistry (Wiley, Hoboken, 2007). https://doi.org/10.1002/9783527610426.bard040502
G.D. Camila, A.F. Galio, Corrosion inhibitors—principles, mechanisms and applications. IntechOpen (2014). https://doi.org/10.5772/57255
S. Fouda, M.A. Migahed, A.A. Atia, I.M. Mousa, Corrosion inhibition and adsorption behavior of some cationic surfactants on carbon steel in hydrochloric acid solution. J. Bio. Tribo. Corros. 2, 22 (2016). https://doi.org/10.1007/s40735-016-0052-1
M. Larouj, K. Ourrak, M. El M’Rabet, H. Zarrok, H. Serrar, M.S. Boudalia, S. Boukhriss, I. Warad, H. Oudda, R. Touir, Thermodynamic study of corrosion inhibition of carbon steel in acidic solution by new pyrimidothiazine derivative. J. Mater. Environ. Sci. 8(11), 3921–3931 (2017)
R.T. Loto, C.A. Loto, Anti-corrosion properties of the symbiotic effect of Rosmarinus officinalis and trypsin complex on medium carbon steel. Results Phys. 10, 99–106 (2018). https://doi.org/10.1016/j.rinp.2018.05.028
Acknowledgments
The author recognizes the support given by Covenant University Ota, Ogun State, Nigeria towards the sponsorship, implementation and successful completion of the research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Loto, R.T., Leramo, R. & Oyebade, B. Synergistic Combination Effect of Salvia officinalis and Lavandula officinalis on the Corrosion Inhibition of Low-Carbon Steel in the Presence of SO42−- and Cl−-Containing Aqueous Environment. J Fail. Anal. and Preven. 18, 1429–1438 (2018). https://doi.org/10.1007/s11668-018-0535-0
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11668-018-0535-0