Abstract
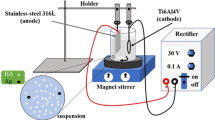
The biomedical sector requires the use of composite materials whose properties can replace those of defective organs, especially bones, of which the Hydroxyapatite structure is the main component of bones and teeth. Herein, we report successful elaboration of new Mg-rich sulfato-calcium hydroxyapatite (SCHA) coating by cathodic electrodeposition on an anodized Ti6Al4V alloy substrate under bias voltages of 10, 20 and 30 V. The resulting deposits were well identified by numerous characterization techniques, such as X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FTIR), Raman spectroscopy and scanning electron microscopy (SEM). Anodizing the Ti-based substrate under a bias voltage of 20 V produces TiO2 layers with ordered nanotube arrays of approximately (80-100 nm), which are completely damaged at 30 V, leading to an irregular porous structure. The deposits made at 10 and 20 V present cracks, unlike that made at 30 V which is more uniform and less rough. XRD analysis reveals both an amorphous structure for the latter and the existence of a crystalline to amorphous conversion as a function of the anodization voltage for the apatite deposition. Electrochemical tests performed in Hank’s simulated body fluid (HSBF) confirm the formation of the apatite deposit which implies a chemical surface modification and reveal interesting anticorrosion properties for the apatite-coated samples, with the highest polarization resistance recorded for that treated at 20 V, with a corrosion current density (icorr) of 4.25 µA/cm2 and a polarization resistance (Rp) of 4.60 kΩ cm2. The results obtained confirm both the nature and the structure of the produced deposits (TiO2 layers and Mg-SCHA/TiO2). Electrochemical tests performed in Hank’s simulated body fluid (HSBF) reveal interesting anticorrosion properties for coated and anodized samples, compared to those uncoated, with the highest corrosion resistance recorded for the anodized sample at 10 V. This suggests that the biocompatible Mg-rich SCHAs deposit, elaborated by simple chemical route from biological waste could be suitable for biomedical purposes.












Similar content being viewed by others
References
W. Nicholson and J. Titanium, Alloys for Dental Implants: A Review, Prosthesis, 2020, 2, p 11.
V. Injeti, K. Nune, E. Reyes, G. Yue, S. Li, and R. Misra, A Comparative Study on the Tribological Behavior of Ti-6Al-4V and Ti-24Nb-4Zr-8Sn Alloys in Simulated Body Fluid, Mater. Technol., 2019, 34, p 270–284.
K. Nune, R. Misra, S. Gaytan, and L. Murr, Biological Response of Next-Generation of 3D Ti-6Al-4V Biomedical Devices Using Additive Manufacturing of Cellular and Functional Mesh Structures, J Biomater Tissue Eng, 2014, 4, p 755–771.
K. Nune, A. Kumar, R. Misra, S. Li, Y. Hao, and R. Yang, Functional Response of Osteoblasts in Functionally Gradient Titanium Alloy Mesh Arrays Processed by 3D Additive Manufacturing, Colloids Surf., B, 2017, 150, p 78–88.
T. Akahori and M. Niinomi, Fracture Characteristics of Fatigued Ti-6Al-4V ELI as an Implant Material, Mater. Sci. Eng., A, 1998, 243, p 237–243.
C. Veiga, J. Davim, and A. Loureiro, Properties and Applications of Titanium Alloys: A Brief Review, Rev. Adv. Mater. Sci., 2012, 32, p 133–148.
K.M. Agarwal, A. Singhal, A. Kapoor, and D. Bhatia, Simulated Analysis of Ti-6Al-4V Processed Through Equal Channel Angular Pressing for Biomedical Applications, Mater. Sci. Energy Technol., 2021, 4, p 290–295.
H.J. Haydar, J. Al-Deen, A.K. AbidAli, and A.A. Mahmoud, Improved Performance of Ti6Al4V Alloy in Biomedical Applications - Review, J. Phys. Conf. Ser., 2021, 1973(1), p 012146. https://doi.org/10.1088/1742-6596/1973/1/012146
P. Amaravathy, S. Sathyanarayanan, S. Sowndarya, and N. Rajendran, Bioactive HA/TiO2 Coating on Magnesium Alloy for Biomedical Applications, Ceram. Int., 2014, 40, p 6617–6630.
M. Jayapriya and M. Arulmozhi, Beta Vulgaris Peel Extract Mediated Synthesis of Ag/TiO2 Nanocomposite: Characterization, Evaluation of Antibacterial and Catalytic Degradation of Textile Dyes-An Electron Relay Effect, Inorg. Chem. Commun., 2021, 128, p 108529.
S.A. Fadl-allah, M. Quahtany, and N.S. El-Shenawy, Surface Modification of Titanium Plate with Anodic Oxidation and Its Application in Bone Growth, J. Biomater. Nanobiotechnol., 2013, 04, p 74–83.
V. PeŠŠková, D. Kubies, H. Hulejova, and L. Himmlova, The Influence of Implant Surface Properties on Cell Adhesion and Proliferation, J. Mater. Sci. Mater. Med., 2007, 18, p 465–473.
H.R. Bakhsheshi-Rad, M. Daroonparvar, M.A.M. Yajid, P. Kumar, M. Razzaghi, A.F. Ismail et al., Characterization and Corrosion Behavior Evaluation of Nanostructured TiO2 and Al2O3-13 wt.%TiO2 Coatings on Aluminum Alloy Prepared via High-Velocity Oxy-Fuel Spray, J. Mater. Eng. Perform., 2021, 30(2), p 1356–1370. https://doi.org/10.1007/s11665-020-05333-4
C. Shan, X. Hou, and K.-L. Choy, Corrosion Resistance of TiO2 Films Grown on Stainless Steel by Atomic Layer Deposition, Surf. Coat. Technol., 2008, 202, p 2399–2402.
P.M. Dziewoński and M. Grzeszczuk, Deposition of thin TiO2 Layers on Platinum by Means of Cyclic Voltammetry of Selected Complex Ti(IV) Media Leading to Anatase, Electrochim. Acta, 2009, 54, p 4045–4055.
D. Atmani, N. Saoula, A. Abdi, M. Azzaz, Y. Wang, and M. Mohamedi, Structural, Morphological, and Electrochemical Corrosion Properties of TiO2 Formed on Ti6Al4V Alloys by Anodization, Cryst. Res. Technol., 2018, 53, p 1800138.
D.P. de Oliveira, T.V. Toniato, R. Ricci, F.R. Marciano, E. Prokofiev, R.Z. Valiev et al., Biological Response of Chemically Treated Surface of the Ultrafine-Grained Ti–6Al–7Nb Alloy for Biomedical Applications, Int. J. Nanomed., 2019, 14, p 1725.
L. Le Guéhennec, A. Soueidan, P. Layrolle, and Y. Amouriq, Surface Treatments of Titanium Dental Implants for Rapid Osseointegration, Dent. Mater., 2007, 23, p 844–854.
C. Legrand-Buscema, C. Malibert, and S. Bach, Elaboration and Characterization of Thin Films of TiO2 Prepared by Sol–Gel Process, Thin Solid Films, 2002, 418, p 79–84.
N. Madaoui, N. Saoula, L. Zougar, I. Djabrouhou, S. Sali, and S. Kermadi, Effect of TiO2 Coating Thickness on the Structure, Mechanical Properties, and Corrosion Behavior of AISI 304L Stainless Steel, J. Mater. Eng. Perform., 2022, 32(2), p 895–908. https://doi.org/10.1007/s11665-022-07144-1
A. Hernández-Gordillo, A. Hernández-Arana, A. Campero-Celis, and L.I. Vera-Robles, TiBALDH as a Precursor for Biomimetic TiO2 Synthesis: Stability Aspects in Aqueous Media, RSC Adv., 2019, 9, p 34559–34566.
D. Rafieian, W. Ogieglo, T. Savenije, and R.G.H. Lammertink, Controlled Formation of Anatase and Rutile TiO2 Thin Films by Reactive Magnetron Sputtering, AIP Adv., 2015 https://doi.org/10.1063/1.4931925
S.N. Atmani Djamila, A. Abderrazak, B. Hadjer, and A. Mohamed. Effect of Electrolyte on the ELlectrochemical and Structural Properties of the TiO2 Layers Elaborated by Anodization. Proceeding paper, 2019.
S. Ali, M. Irfan, U.M. Niazi, A.M.A. Rani, A. Rashedi, S. Rahman et al., Microstructure and Mechanical Properties of Modified 316L Stainless Steel Alloy for Biomedical Applications Using Powder Metallurgy, Materials, 2022, 15, p 2822.
M.S. Gogheri, M. Kasiri-Asgarani, H.R. Bakhsheshi-Rad, H. Ghayour, and M. Rafiei, In Vitro Corrosion Behavior and Cytotoxicity of Polycaprolactone–Akermanite-Coated Friction-Welded Commercially Pure Ti/AZ31 for Orthopedic Applications, J. Mater. Eng. Perform., 2020, 29, p 6053–6065.
H. Bakhsheshi-Rad, E. Hamzah, M. Abdul-Kadir, S.N. Saud, M. Kasiri-Asgarani, and R. Ebrahimi-Kahrizsangi, The Mechanical Properties and Corrosion Behavior of Double-Layered Nano Hydroxyapatite-Polymer Coating on Mg-Ca Alloy, J. Mater. Eng. Perform., 2015, 24, p 4010–4021.
S. Ahmadi, I. Mohammadi, and S. Sadrnezhaad, Hydroxyapatite Based and Anodic Titania Nanotube Biocomposite Coatings: Fabrication, Characterization and Electrochemical Behavior, Surf. Coat. Technol., 2016, 287, p 67–75.
J. Lu, G. Wei, Y. Yu, X. Zhao, and Y. Dai, Enhanced Corrosion Resistance of TA2 Titanium Via Anodic Oxidation in Mixed Acid System, Int. J. Electrochem. Sci., 2017, 12, p 2763–2776.
O.V. Tkachuk, I.M. Pohrelyuk, R.V. Proskurnyak, J. Morgiel, M. Faryna, and A. Goral, Morphology and Corrosion Resistance of Hydroxyapatite Coatings Formed on Commercially Pure Titanium, J. Mater. Eng. Perform., 2023, 32(24), p 11040–11049. https://doi.org/10.1007/s11665-023-07910-9
D. He, P. Liu, X. Liu, X. Chen, F. Ma, W. Li et al., Hydroxyapatite Bioceramic Coatings Prepared by Hydrothermal-Electrochemical Deposition Method, J. Wuhan Univ. Technol. Mater. Sci. Ed., 2014, 29, p 398–400.
L. Benea, E. Mardare-Danaila, M. Mardare, and J.-P. Celis, Preparation of Titanium Oxide and Hydroxyapatite on Ti–6Al–4V Alloy Surface and Electrochemical Behaviour in Bio-Simulated Fluid Solution, Corros. Sci., 2014, 80, p 331–338.
J. Heughebaert, S. Zawacki, and G. Nancollas, The Growth of Nonstoichiometric Apatite from Aqueous Solution at 37 °C: I. Methology and Growth at pH 7.4, J. Colloid Interface Sci., 1990, 135, p 20–32.
Y. Jie Zhao, W.-bS. Liu, and H. Zhang, Amorphous Calcium Phosphate and Its Application in Dentistry, Chem. Central J., 2011 https://doi.org/10.1186/1752-153X-5-40
B. Jin, Z. Liu, C. Shao, J. Chen, L. Liu, R. Tang et al., Phase Transformation Mechanism of Amorphous Calcium Phosphate to Hydroxyapatite Investigated by Liquid-Cell Transmission Electron Microscopy, Cryst. Growth Des., 2021, 21, p 5126–5134.
V. Tazzoli, The Crystal Structure of Cesanite, Ca1+xNa4−x (SO4)3(OH)x(1–x)H2O, a Sulphate Isotypic to Apatite, Min. Mag., 1983, 47, p 59–63.
T. Toyama, S. Kameda, and N. Nishimiya, Synthesis of Sulfate-ion-Substituted Hydroxyapatite from Amorphous Calcium Phosphate, Bioceram. Dev. Appl. S, 2013, 1, p 2.
E. Fiume, G. Magnaterra, A. Rahdar, E. Verné, and F. Baino, Hydroxyapatite for Biomedical Applications: A Short Overview, Ceramics, 2021, 4, p 542–563.
M.R. Senra, R.B. de Lima, D.H.S. Souza, M.F.V. Marques, and S.N. Monteiro, Thermal Characterization of Hydroxyapatite or Carbonated Hydroxyapatite Hybrid Composites with Distinguished Collagens for Bone Graft, J. Mater. Res. Technol., 2020, 9, p 7190–200.
M. Rondanelli, M.A. Faliva, A. Tartara, C. Gasparri, S. Perna, V. Infantino et al., An Update on Magnesium and Bone Health, Biometals, 2021, 34, p 715–736.
C. Garbo, J. Locs, M. D’Este, G. Demazeau, A. Mocanu, C. Roman et al., Advanced Mg, Zn, Sr, Si Multi-Substituted Hydroxyapatites for Bone Regeneration, Int. J. Nanomed., 2020, 15, p 1037–1058. https://doi.org/10.2147/IJN.S226630
L. Bauer, M. Ivanković, H. Ivanković. Magnesium substituted hydroxyapatite scaffolds hydrothermally synthesized from cuttlefish bone magnezijem supstituirani hidroksiapatitni nosači hidrotermalno sintetizirani iz sipine kosti.
A. Piotrowski, V. Kahlenberg, R. Fischer, Y. Lee, and J. Parise, The Crystal Structures of Cesanite and Its Synthetic Analogue—A Comparison, Am. Miner., 2002, 87, p 715–720.
L. Stipniece, K. Salma-Ancane, N. Borodajenko, M. Sokolova, D. Jakovlevs, and L. Berzina-Cimdina, Characterization of Mg-Substituted Hydroxyapatite Synthesized by Wet Chemical Method, Ceram. Int., 2014, 40, p 3261–3267.
M.S. Hossain, M.A.A. Shaikh, and S. Ahmed, Synthesis of Gypsum Fertilizer from Waste Eggshells for a Sustainable Environment, Mater. Adv., 2023, 4, p 240–247.
N. Tangboriboon, W. Unjan, W. Sangwan, and A. Sirivat, Preparation of Anhydrite from Eggshell Via Pyrolysis, Green Process. Synth., 2018, 7, p 139–146.
S. Naidu and G.W. Scherer. Development of hydroxyapatite films to reduce the dissolution rate of marble. In Proceedings of 12th International Congress on Deterioration and Conservation of Stone, New York City (USA), 2012, p 1–9.
S.B. Shinde, S.R. Bhosale, N.B. Birajdar, A.H. Gore, G.B. Kolekar, S.S. Kolekar et al., Construction of Waste Chalk Powder into mpg-C3N4-CaSO4 as an Efficient Photocatalyst for Dye Degradation under UV–Vis Light and Sunlight, Langmuir, 2023, 39, p 6324–6336.
M. Kosmulski, Surface Charging and Points of Zero Charge, CRC Press, Cambridge, 2009.
Z. Chen, H. Liu, X. Liu, X. Lian, Z. Guo, H.-J. Jiang et al., Improved Workability of Injectable Calcium Sulfate Bone Cement by Regulation of Self-Setting Properties, Mater. Sci. Eng. C, 2013, 33, p 1048–1053.
D.L. Goloshchapov, V.M. Kashkarov, N.A. Rumyantseva, P.V. Seredin, A.S. Lenshin, B.L. Agapov et al., Synthesis of Nanocrystalline Hydroxyapatite by Precipitation Using Hen’s Eggshell, Ceram. Int., 2013, 39(4), p 4539–4549. https://doi.org/10.1016/j.ceramint.2012.11.050
F. Rosi, A. Daveri, B. Doherty, S. Nazzareni, B.G. Brunetti, A. Sgamellotti et al., On the Use of Overtone and Combination Bands for the Analysis of the CaSO4–H2O System by Mid-Infrared Reflection Spectroscopy, Appl. Spectrosc., 2010, 64, p 956–963.
N.T.N. Le, N.T.T. Le, Q.L. Nguyen, T.L.-B. Pham, M.-T. Nguyen-Le, and D.H. Nguyen, A facile synthesis process and evaluations of α-calcium sulfate hemihydrate for bone substitute, Materials, 2020, 13(14), p 3099. https://doi.org/10.3390/ma13143099
S. Pramanik, A.K. Agarwal, K. Rai, and A. Garg, Development of High Strength Hydroxyapatite by Solid-State-Sintering Process, Ceram. Int., 2007, 33, p 419–426.
J.M. Cao, J. Feng, S.G. Deng, X. Chang, J. Wang, J.S. Liu et al., Microwave-Assisted Solid-State Synthesis of Hydroxyapatite Nanorods at Room Temperature, J. Mater. Sci., 2005, 40, p 6311–6313.
R. Jenkins and R.L. Snyder, Introduction to X‐ray Powder Diffractometry, Wiley, 1996. https://doi.org/10.1002/9781118520994
A.R. Noviyanti, N. Akbar, Y. Deawati, E.E. Ernawati, Y.T. Malik, and R.P. Fauzia, A Novel Hydrothermal Synthesis of Nanohydroxyapatite from Eggshell-Calcium-Oxide Precursors, Heliyon, 2020, 6, p e03655.
T. Schmid, R. Jungnickel, and P. Dariz, Insights Into the CaSO4–H2O System: A Raman-Spectroscopic Study, Minerals, 2020, 10, p 115.
N. Prieto-Taboada, O. Gomez-Laserna, I. Martínez-Arkarazo, M.Á. Olazabal, and J.M. Madariaga, Raman Spectra of the Different Phases in the CaSO4–H2O System, Anal. Chem., 2014, 86, p 10131–10137.
K. Ben Mabrouk, T.H. Kauffmann, H. Aroui, and M.D. Fontana, Raman Study of Cation Effect on Sulfate Vibration Modes in Solid State and in Aqueous Solutions, J. Raman Spectrosc., 2013, 44, p 1603–1608.
E.J. Ekoi, A. Gowen, R. Dorrepaal, and D.P. Dowling, Characterisation of Titanium Oxide Layers Using Raman Spectroscopy and Optical Profilometry: Influence of Oxide Properties, Results Phys., 2019, 12, p 1574–1585.
M. Meininger, S. Meininger, J. Groll, U. Gbureck, and C. Moseke, Silver and Copper Addition Enhances the Antimicrobial Activity of Calcium Hydroxide Coatings on Titanium, J. Mater. Sci. Mater. Med., 2018, 29, p 1–9.
M.O. Li, X. Xiao, R. Liu, C. Chen, and L. Huang, Structural Characterization of Zinc-Substituted Hydroxyapatite Prepared by Hydrothermal Method, J. Mater. Sci. - Mater. Med., 2008, 19, p 797–803.
L. Degli Esposti and M. Iafisco, Amorphous Calcium Phosphate, the Lack of Order is an Abundance of Possibilities, Biomater. Biosyst., 2022, 5, p 100037.
P. Wolint, L. Näf, D. Schibler, N. Hild, W.J. Stark, P. Giovanoli et al., Suspension of Amorphous Calcium Phosphate Nanoparticles Impact Commitment of Human Adipose-Derived Stem Cells In Vitro, Biology, 2021, 10, p 675.
R. Shendrik, E. Kaneva, T. Radomskaya, I. Sharygin, and A. Marfin, Relationships Between the Structural, Vibrational, and Optical Properties of Microporous Cancrinite, Crystals, 2021, 11, p 280.
D. Govindaraj, M. Rajan, M.A. Munusamy, and A. Higuchi, Mineral Substituted Hydroxyapatite Coatings Deposited on Nanoporous TiO2 Modulate the Directional Growth and Activity of Osteoblastic Cells, RSC Adv., 2015, 5, p 58980–58988.
W.L. Suchanek, K. Byrappa, P. Shuk, R.E. Riman, V.F. Janas, and K.S. TenHuisen, Preparation of Magnesium-Substituted Hydroxyapatite Powders by the Mechanochemical–Hydrothermal Method, Biomaterials, 2004, 25, p 4647–4657.
R.K. Rude, Magnesium Deficiency: A Cause of Heterogenous Disease in Humans, J. Bone Miner. Res., 1998, 13, p 749–758.
Y. Matsumoto, H. Harada, K. Yui, H. Uchida, K. Itatani, and S. Koda, Raman Spectroscopic Study of Aqueous Alkali Sulfate Solutions at High Temperature and Pressure to Yield Precipitation, J. Supercrit. Fluids, 2009, 49, p 303–309.
L.K. Tran, K.R. Stepien, M.M. Bollmeyer, and C.H. Yoder, Substitution of Sulfate in Apatite, Am. Min. J. Earth Planet. Mater., 2017, 102, p 1971–1976.
W. Rudolph, D. Fischer, G. Hefter, and G. Irmer, Raman Spectroscopic Investigation of Speciation in MgSO4 (aq), Sci. Access, 2004, 2, p 146–147.
M. Lubas, J. Jasinski, M. Sitarz, L. Kurpaska, P. Podsiad, and J. Jasinski, Raman Spectroscopy of TiO2 Thin Films Formed by Hybrid Treatment for Biomedical Applications, Spectrochim. Acta Part A Mol. Biomol. Spectrosc., 2014, 133, p 867–871.
X. Xia, S. Peng, Y. Bao, Y. Wang, B. Lei, Z. Wang et al., Control of Interface Between Anatase TiO2 Nanoparticles and Rutile TiO2 Nanorods for Efficient Photocatalytic H2 Generation, J. Power Sources, 2018, 376, p 11–17.
L.-Y. Chen, H.-Y. Zhang, C. Zheng, H.-Y. Yang, P. Qin, C. Zhao et al., Corrosion Behavior and Characteristics of Passive films of Laser Powder Bed Fusion Produced Ti–6Al–4V in Dynamic Hank’s Solution, Mater. Des., 2021, 208, p 109907.
M.G.R. Mahlobo, L. Chikosha, and P.A. Olubambi, Study of the Corrosion Properties of Powder Rolled Ti–6Al–4V Alloy Applied in the Biomedical Implants, J. Mater. Res. Technol., 2022, 18, p 3631–3639. https://doi.org/10.1016/j.jmrt.2022.04.004
N. Dai, J. Zhang, Y. Chen, and L.-C. Zhang, Heat Treatment Degrading the Corrosion Resistance of Selective Laser Melted Ti-6Al-4V alloy, J. Electrochem. Soc., 2017, 164, p C428.
Y. Otsuka, H. Kawaguchi, and Y. Mutoh, Cyclic Delamination Behavior of Plasma-Sprayed Hydroxyapatite Coating on Ti–6Al–4V Substrates in Simulated Body Fluid, Mater. Sci. Eng., C, 2016, 67, p 533–541.
Y.-W. Cui, L.-Y. Chen, Y.-H. Chu, L. Zhang, R. Li, S. Lu et al., Metastable Pitting Corrosion Behavior and Characteristics of Passive Film of Laser Powder Bed Fusion Produced Ti–6Al–4V in NaCl Solutions with Different Concentrations, Corros. Sci., 2023, 215, p 111017.
Z. Li, W. Zhao, G. Xiao, K. Chen, H. Zhang, N. Guo et al., Impact of Microstructure Evolution on the Corrosion Behaviour of the Ti–6Al–4V Alloy Welded Joint Using High-Frequency Pulse Wave Laser, J. Market. Res., 2023, 24, p 4300–4314.
M. Cabrini, A. Carrozza, S. Lorenzi, T. Pastore, C. Testa, D. Manfredi et al., Influence of Surface Finishing and Heat Treatments on the Corrosion Resistance of LPBF-Produced Ti-6Al-4V Alloy for Biomedical Applications, J. Mater. Process. Technol., 2022, 308, p 117730.
S. Khanmohammadi, M. Ojaghi-Ilkhchi, and M. Farrokhi-Rad, Development of Bioglass Coating Reinforced with Hydroxyapatite Whiskers on TiO2 Nanotubes Via Electrophoretic Deposition, Ceram. Int., 2021, 47, p 1333–1343.
V. Simi and N. Rajendran, Influence of Tunable Diameter on the Electrochemical Behavior and Antibacterial Activity of Titania Nanotube arrays for Biomedical Applications, Mater. Charact., 2017, 129, p 67–79.
M. Galvan-Ruiz, L. Banos, and M.E. Rodriguez-Garcia, Lime Characterization as a Food Additive, Sens. Instrum. Food Qual. Saf., 2007, 1, p 169–175.
S. Tanpure, V. Ghanwat, B. Shinde, K. Tanpure, and S. Lawande, The Eggshell Waste Transformed Green and Efficient Synthesis of K-Ca (OH)2 Catalyst for Room Temperature Synthesis of Chalcones, Polycyclic Aromat. Compd., 2022, 42, p 1322–1340.
M. Belhabra, S. Zerraf, A. Kheireddine, I. Fahim, M. Tridane, A. Abouimrane et al., Vibrational Study, Reinvestigation of the Crystal Structure of MgHPO4·3H2O and Calculated IR Frequencies for the PO4 3-by Isotopic Substitutions, Biointerface Res. Appl. Chem., 2021, 12(3), p 4140–4154.
I. Škugor Rončević, N. Vladislavić, M. Buzuk, and M. Buljac, Electrodeposition of Hydroxyapatite Coating on Mg Alloy Modified with Organic Acid Self-Assembled Monolayers, J. Chem. Res., 2020, 44, p 212–220.
Acknowledgment
The authors would like to thank both the Military Polytechnic School and the Center for Development of Advanced Technologies for providing the financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no financial or commercial conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Atmani, D., Saoula, N., Chouchane, K. et al. Study of Mg-Rich Sulfato-Calcium Hydroxyapatite Coating on Anodized Ti6Al4V-Alloy for Biomedical Applications. J. of Materi Eng and Perform (2024). https://doi.org/10.1007/s11665-024-09492-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11665-024-09492-6