Abstract
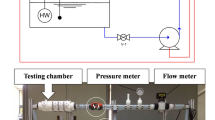
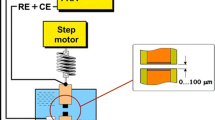
Corrosion is a major problem in cooling water systems, which is often controlled using corrosion inhibitors. Solution hydrodynamics is one of the factors affecting corrosion inhibition of metals in these systems. The present work focuses on the study of the combined effects of citric acid concentration (as a green corrosion inhibitor) and fluid flow on corrosion of steel in simulated cooling water. Electrochemical techniques including Tafel polarization and electrochemical impedance spectroscopy were used for corrosion studies. Laminar flow was simulated using a rotating disk electrode. The effects of solution hydrodynamics on inhibition performance of citric acid were discussed. The citric acid showed low inhibition performance in quiescent solution; however, when the electrode rotated at 200 rpm, inhibition efficiency increased remarkably. It was attributed mainly to the acceleration of inhibitor mass transport toward metal surface. The efficiencies were then decreased at higher rotation speeds due to enhanced wall shear stresses on metal surface and separation of adsorbed inhibitor molecules. This article is first part of authors’ attempts in designing green inhibitor formulations for industrial cooling water. Citric acid showed acceptable corrosion inhibition in low rotation rates; thus, it can be used as a green additive to the corrosion inhibitor formulations.







Similar content being viewed by others
References
J. Marín-Cruz, R. Cabrera-Sierra, M.A. Pech-Canul, and I. González, EIS Study on Corrosion and Scale Processes and Their Inhibition in Cooling System Media, Electrochim. Acta, 2006, 51, p 1847–1854
S. Ramesh, S. Rajeswari, and S. Maruthamuthu, Corrosion Inhibition of Copper by New Triazole Phosphonate Derivatives, Appl. Surf. Sci., 2004, 229, p 214–225
P.R. Roberge, Handbook of Corrosion Engineering, McGraw-Hill, New York, 2000
A. Abulkibash, M. Khaled, B. El Ali, and M. Emad, Corrosion Inhibition of Steel in Cooling Water System by 2-phosphonobutane 1,2,4-tricarboxylic acid and Polyvinylpyrrolidone, Arab. J. Sci. Eng., 2008, 33, p 29–40
A.M. Badiea and K.N. Mohana, Corrosion Mechanism of Low-Carbon Steel in Industrial Water and Adsorption Thermodynamics in the Presence of Some Plant Extracts, J. Mater. Eng. Perform., 2009, 18, p 1264–1271
A. Buyuksagis and S. Erol, The Examination of Afyonkarahisar’s Geothermal System Corrosion, J. Mater. Eng. Perform., 2013, 22, p 563–573
N. Dkhireche, A. Dahami, A. Rochdi, J. Hmimou, R. Touir, M. Ebn Touhami, M. El Bakri, A. El Hallaoui, A. Anouar, H. Takenouti, Corrosion and Scale Inhibition of Low Carbon Steel in Cooling Water System by 2-propargyl-5-o-hydroxyphenyltetrazole, J. Ind. Eng. Chem., 2013, 19, p 1996–2003
R. Touir, N. Dkhireche, M. Ebn Touhami, M. Lakhrissi, B. Lakhrissi, M. Sfaira, Corrosion and Scale Processes and Their Inhibition in Simulated Cooling Water Systems by Monosaccharides Derivatives, Part I: EIS Study, Desalination, 2009, 249, p 922–928
D.Z. Cai, G. Sang, J.X. Liu, J.R. Zhong, and D.M. Lang, Corrosion Inhibition Behavior of Different Inhibitors for Depleted Uranium in Cooling Water, Atomic Energy Sci. Technol., 2012, 46, p 158–163
I. Sekine, M. Sanbongi, H. Hagiuda, T. Oshibe, M. Yuasa, T. Imahama, Y. Shibata, and T. Wake, Corrosion Inhibition of Mild Steel by Cationic and Anionic Polymers in Cooling Water System, J. Electrochem. Soc., 1992, 139, p 3167–3173
C.M. Mustafa and M.M. Haque, Corrosion Inhibition of Aluminium by Molybdate, Citric Acid, and Nitrite in Simulated Cooling Water, Corros. Prev. Control, 1997, 44, p 49–55
X.M. Xu, H.H. Ge, J.N. Wu, R. Sun, J.X. Han, H.L. Xu, and Z.Z. Liu, Corrosion Inhibition of Sodium Dodecyl Benzene Sulfonate to Stainless Steel, Corros. Protect., 2012, 33, p 740–743
M. Saremi, C. Dehghanian, and M. Mohammadi Sabet, The Effect of Molybdate Concentration and Hydrodynamic Effect on Mild Steel Corrosion Inhibition in Simulated Cooling Water, Corros. Sci., 2008, 48, p 1404–1412
M. Salasi, T. Shahrabi, and E. Roayaei, Effect of Inhibitor Concentration and Hydrodynamic Conditions on the Inhibitive Behaviour of Combinations of Sodium Silicate and HEDP for Corrosion Control in Carbon Steel Water Transmission Pipes, Anti Corros. Methods Mater., 2007, 54, p 82–92
A.M. Badiea and K.N. Mohana, Effect of Temperature and Fluid Velocity on Corrosion Mechanism of Low Carbon Steel in Presence of 2-hydrazino-4,7-dimethylbenzothiazole in Industrial Water Medium, Corros. Sci., 2009, 51, p 2231–2241
A.M. Badiea and K.N. Mohana, Effect of Fluid Velocity and Temperature on the Corrosion Mechanism of Low Carbon Steel in Industrial Water in the Absence and Presence of 2-hydrazino benzothiazole, Korean J. Chem. Eng., 2008, 25, p 1292–1299
F.H. Verhoff, Citric acid, Ullmann’s Encyclopedia of Industrial Chemistry, W. Gerhartz, Ed., Wiley-VCH, Weinheim, 2005,
R. Solmaz, G. Kardaş, B. Yazıcı, and M. Erbil, Citric Acid as Natural Corrosion Inhibitor for Aluminium Protection, Corros. Eng. Sci. Technol., 2008, 43, p 186–191
D.J. Choi, S.J. You, and J.G. Kim, Development of an Environmentally Safe Corrosion, Scale, and Microorganism Inhibitor for Open Recirculating Cooling Systems, Mater. Sci. Eng. A, 2002, 335, p 228–235
B. Müller, W. Kläger, and G. Kubitzki, Metal Chelates of Citric Acid as Corrosion Inhibitors for Zinc Pigment, Corros. Sci., 1997, 39, p 1481–1485
F.M. Mahgoub, B.A. Abdel-Nabey, and Y.A. El-Samadisy, Adopting a Multipurpose Inhibitor to Control Corrosion of Ferrous Alloys in Cooling Water Systems, Mater. Chem. Phys., 2010, 120, p 104–108
G. Kear, B.D. Barker, K.R. Stokes, and F.C. Walsh, Electrochemistry of Non-Aged 90-10 Copper-Nickel Alloy (UNS C70610) as a Function of Fluid Flow, Part 1: Cathodic and Anodic Characteristics, Electrochim. Acta, 2007, 52, p 1889–1898
N. Perez, Electrochemistry and Corrosion Science, Kluwer, Norwell, 2004
J.A. Wharton and R.J.K. Wood, Influence of Flow Conditions on the Corrosion of AISI, 304L Stainless Steel, Wear, 2004, 256, p 525–536
H. Ashassi-Sorkhabi and E. Asghari, Influence of Flow on the Corrosion Inhibition of St52-3 Type Steel by Potassium Hydrogen-Phosphate, Corros. Sci., 2009, 51, p 1828–1835
W.S. Tait, An Introduction to Electrochemical Corrosion Testing for Practicing Engineers and Scientists, Paris O Docs Publications, New York, 1994
A.J. Francis and C.J. Dodge, Influence of Complex Structure on the Biodegradation of Iron-Citrate Complexes, Appl. Environ. Microbiol., 1993, 59, p 109–113
L. Königsberger, E. Königsberger, P.M. May, and G.T. Hefter, Complexation of Iron (III) and Iron (II) by Citrate. Implications for Iron Speciation in Blood Plasma, J. Inorg. Biochem., 2000, 78, p 175–184
A.B. Hastings, F.C. McLean, L. Eichelberger, J. Lowell Hall, and E. Da Costa, The Ionization of Calcium, Magnesium and Stronsium Citrates, J. Biol. Chem., 1934, 107, p 351–370
T.B. Field, J.L. McCourt, and W.A.E. McBryde, Composition and Stability of Iron and Copper Citrate Complexes in Aqueous Solution, Can. J. Chem., 1974, 52, p 3119–3123
W.R. Osório, E.S. Freitas, and A. Garcia, Corrosion Performance Based on the Microstructural Array of Al-Based Monotectic Alloys in a NaCl Solution, J. Mater. Eng. Perform., 2014, 23, p 333–341
W.R. Osório, E.S. Freitas, and A. Garcia, EIS and Potentiodynamic Polarization Studies on Immiscible Monotectic Al-In Alloys, Electrochim. Acta, 2013, 102, p 436–445
Acknowledgment
The authors would like to thank University of Tabriz for financial support of the project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ashassi-Sorkhabi, H., Asghari, E. & Mohammadi, M. Effects of Solution Hydrodynamics on Corrosion Inhibition of Steel by Citric Acid in Cooling Water. J. of Materi Eng and Perform 23, 2992–3000 (2014). https://doi.org/10.1007/s11665-014-1047-z
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11665-014-1047-z