Abstract
Using a mixture of micro-copper and nano-silver in the production of screen-printed circuits has the potential to reduce material costs and cost variability. The fundamental premise of this study involved dispersing silver nanoparticles among the larger copper microparticles at selected ratios and subsequently sintering in order to establish their resultant electrical and physical performance. Commercial materials were mixed, printed, and sintered at two thermal regimes on fluorine-doped tin oxide (FTO)-coated glass substrate. The inclusion of 25% silver provided an appreciable reduction in electrical resistance from 4.21 Ω to 0.93 Ω, with further silver additions having less impact. The thermal regime used for sintering had a secondary impact on the final electrical performance. The addition of silver reduced the adhesion to the FTO substrate, with reduced film integrity. The results show that blending inks offers the advantage of enhancing material conductivity while simultaneously reducing costs, making it a compelling area for exploration and advancement in the field of electronics manufacturing.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The conductive circuit serves as an essential element in every electrical or electronic device.1,2 Although silver boasts excellent conductivity, stability in organic suspensions, and a commendable life span, its relatively high cost and susceptibility to price fluctuations are drawbacks.3,4 Copper ink, with its demonstrated applications in radio-frequency identification (RFID) aerials, hybrid silicon/printed flexible circuits, and thin film transistor electrodes, emerges as a promising alternative material.5
These applications focus primarily on polymer substrates, particularly polyimide, due to its ability to withstand higher sintering temperatures, along with its flexibility and compatibility with reel-to-reel processes. The objective of this study is to investigate a mixed ink consisting of copper nano/microparticles and silver nanoparticles that can be sintered at lower temperatures. This concept draws inspiration from existing literature and the promising outcomes of previous studies utilizing the combination of copper nano/microparticle ink. One advantage of using larger particles in the copper ink is the reduction in oxidation levels due to the lower surface area-to-volume ratio.1,6 Copper nano/microparticle ink typically necessitates high sintering temperatures, which align with the sintering requirements of the nano-silver ink (250–500°C).7 Furthermore, copper ink encounters issues with oxidation during storage and processing, which can be mitigated by blending it with silver. The higher surface area of copper nanoparticles leads to a quicker oxidation rate compared to larger particles. The existing literature has explored the potential of blended ink to decrease sintering temperatures,3 but it has not examined the application on glass substrates coated with a fluorine-doped tin oxide (FTO) surface, which is the substrate of choice for many third-generation photovoltaics (PV), where material costs are critical. Therefore, the goal of this study is to assess the viability of using a silver–copper ink on FTO glass substrates and to determine whether it offers any potential improvements over conventional silver ink.
Methods
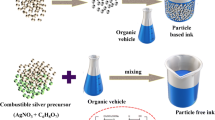
The copper microparticle ink was supplied by Intrinsiq (IMCE4118) and selected from a previous study, as it exhibited the lowest oxidation under H2/N2 atmosphere sintering, provided good conductivity, and was stable, showing no drift in resistance after 4 weeks of sintering. The sintering time and temperature were set within the operational window of copper ink and nano-silver ink. The silver ink was supplied by AGFA (ORGACON) and is a well-established material known for its high conductivity, low deposition, and ability to support high-resolution patterning applications. The 30–40 g blends were mixed manually, and 20 samples were produced from each batch. The mixing was carried out manually with three ratios of silver-copper ink as shown Table I.
The single metal and mixed ink were screen-printed using an ATMA semi-automatic printer, with an image consisting of areas that allowed for characterization of fine lines (200–600 μm wide). The images were printed to Tec-7 FTO-coated glass from NSG (nominal sheet resistance of 7 Ω/sq), with a 110-34 polyester mesh on an automatic ATMA flatbed printing machine. A Lenton model CSC 12/90/600H tube furnace was used, which could be sealed at either end and filled with a sintering atmosphere of a mix of 5% hydrogen and 95% nitrogen. Figure 1 shows the method steps.
After drying for 10 min at 80°C, the samples were subjected to two thermal regimes. The first involved a 30-min treatment at 250°C, which was determined to be the optimum temperature for copper material. The second regime followed the supplier's recommendation for silver ink, specifying a temperature of 275°C for 10 min. The TQC test kit was used in this test to measure the adhesion. A JEOL JSM-7800 scanning electron microscope was used for surface analysis.
Results
At 25% silver content, the adhesive performance of the ink was mostly unaffected, with a small increase in the area removed as the silver content increased to 54% (Table I). As the silver content increased to 75%, more of the film was removed by tape, leading to complete delamination under testing. This behavior is similar to that observed with nano-copper ink, where sintering of copper nanoparticles reduces adhesion. It is believed that the sintering of nanoparticles and formation of a denser conductive film negatively impacts the ink–substrate interface (Table II).
All electrical characteristics showed improved conductivity when the silver content was increased (Fig. 2). The copper 600-micron line resistance was 4.21Ω, while the silver was 0.16Ω (Fig. 2a). There was an initial sudden reduction as silver was added followed by a more gradual decrease as silver content increased further. This was common for both thermal sintering regimes. Similar trends were observed for the sheet resistance, Fig. 2b, contact resistance, Fig. 2c, and absolute resistivity, Fig. 2d.
The inference which can be drawn from these results is that the sintering of the copper provides a less conductive feature than that produced from the silver ink. This is likely a result of the oxidation of the copper material during the wet, drying, or sintering process, resulting in a less densely packed film with microparticles. The film density and ease of a conductive pathway can be seen in Fig. 3, where the silver nanoparticles sinter to form a more continuous film.
X-ray diffraction was used to identify changes in the structure and possible oxidation due to the sintering process. Figure 4 shows the diffraction peaks observed at 2θ 111, 200, and 220 planes in all mixed inks, indicating the presence of copper and silver. The relative intensities of the peaks are in line with the ratios of the silver and copper components. No additional peaks are visible due to the sintering process, indicating little chemical change during the sintering process. Three characteristic copper peaks are clearly present at 2θ values of 43.27°, 53.42, and 74.12, corresponding to (111), (200), and (220), respectively. These characteristic peaks confirm that a face-centered cubic (FCC) copper phase is present. There is also no evidence of the Cu2O phase at 36.6°, which indicates that minimal oxidation has occurred. Also, the presence of peaks at 2θ values of 26.2°, 38.19°, and 44.22° is indicative of a layer of silver, which should provide extra protection to the copper against oxidation.8
Discussion
This study addresses several points that have both scientific and practical implications. Firstly, it demonstrates that incorporating a small amount of nano-silver ink into micro/nano-copper ink can effectively reduce resistance and enhance conductive performance. The addition of 25% nano-silver was shown to decrease sheet and line resistance by up to three times, all while maintaining strong adhesion on FTO-coated glass substrates. This finding offers a cost-effective solution for improving conductivity. Moreover, the study suggests that further optimization of the silver–copper ratio could potentially achieve an even better balance between conductivity and cost. Initial cost estimates suggest an approximately three- to fourfold reduction in the bill of materials. Any such cost analysis would need to examine the additional costs of producing a controlled reducing environment. The blending of silver with copper ink had a positive impact on electrical performance, which was more than could be explained by the lower resistivity of silver alone. It is postulated that the silver improved the conductive structure and pathways of the film, enabling the large copper particles to be the primary backbone of conduction with inter-micro-copper particle junctions being filled with fully sintered silver nanoparticles. The lack of visible nano-copper sintering (Fig. 3a) suggests that the presence of microparticles prevents visible sintering of the copper nanoparticles. However, this phenomenon is not observed with the silver ink (Fig. 3c), and the study proposes that the presence of silver may help prevent copper oxidation during the sintering process. It is also plausible that silver particles react preferentially with any available oxygen, thereby inhibiting the formation of copper oxide.
Conclusion
The work conducted in this study has demonstrated the potential of copper/silver mixtures as a viable method for producing highly conductive and physically durable structures on FTO-coated glass. The study achieved success by formulating a blended ink in which the addition of 25% nano-silver achieved a significant fivefold reduction in conductivity (4.21–0.93 Ω) while still leveraging the potentially lower cost of copper, the essential conductive material. The blending of copper nano/micro-inks with silver nanoparticles offers the advantage of preserving adhesion on FTO substrates while simultaneously enhancing material conductivity. A significant consideration is that achieving precision and uniformity in the deposition of materials at larger scale can be challenging.
Data availability
Data will be made available on request.
References
B. Abbas, Y. Mohammad, E. Jewell, and J. Searle, Thermal sintering of printable copper for enhanced conductivity of FTO coated glass substrates. J. Mater. Sci. 30, 19917–19927 (2019).
A.J. Cobley, B. Abbas, and A. Hussain, Improved electroless copper coverage at low catalyst concentrations and reduced plating temperatures enabled by low frequency ultrasound. Int. J. Electrochem. Sci. 9, 7795–7804 (2014).
E. Jewell, S. Hamblyn, T. Claypole, and D. Gethin, Deposition of high conductivity low silver content materials by screen printing. Coatings 5, 172–185 (2015).
F. De Rossi, J.A. Baker, D. Beynon, K. Hooper, S. Meroni, D. Williams, E. Jewell, T. Watson, C. Charbonneau, A. Yasin, and Z. CommentWei, All printable perovskite solar modules with 198 cm2 active area and over 6% efficiency. Adv. Mater. Technol. 3, 1–9 (2018).
Y. Kim, B. Lee, S. Yang, I. Byun, I. Jeong, and S. Cho, Use of copper ink for fabricating conductive electrodes and RFID antenna tags by screen printing. Curr. Appl. Phys. 12, 473–478 (2012).
B. Abbas, E. Jewell, Y.C. Lau, J. Searle, and T. Claypole, Photonic sintering of copper for rapid processing of thick film conducting circuits on FTO coated glass. Sci. Rep. 13, 1–12 (2023).
Bai G (2005) Low-Temperature sintering of nanoscale silver paste for semiconductor device interconnection. Mater. Sci. 45–48
M. Grouchko, A. Kamyshny, and S. Magdassi, Formation of air-stable copper-silver core-shell nanoparticles for inkjet printing. J. Mater. Chem. 19, 3057–3062 (2009).
Acknowledgments
This work was supported by SPECIFIC Innovation and Knowledge Centre, EPSRC [EP/N020863/1], Innovate UK [920036], EPSRC [EP/N509905/1], and the European Regional Development Fund [c80892] through the Welsh Government. Thanks to Hi-prospects partner companies and institutions, especially Intrinsiq materials for material supply support.
Funding
The Funding was provided by Engineering and Physical Sciences Research Council, EP/N020863/1, Bahaa Abbas
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest. All co-authors have seen and agree with the contents of the manuscript, and there is no financial interest to report.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abbas, B., Jewell, E. & Searle, J. Blended Copper and Nano-Silver Screen-Printed Circuits on FTO-Coated Glass. J. Electron. Mater. 53, 2498–2503 (2024). https://doi.org/10.1007/s11664-024-10962-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11664-024-10962-9