Abstract
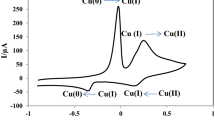
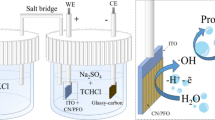
PrCrO3 perovskite-type nanoparticles were prepared by the co-precipitation method. In the hexagonal phase, highly crystalline particles with an average particle size of 80–90 nm were observed from the x-ray diffraction pattern. Transmission electron microscopic images showed irregularly shaped, smooth surfaces, with narrow-size-distributed nanocrystals. Phase purity, thermal stability, and optical activity were monitored by Fourier transform infrared spectroscopy, thermogravimetric analysis, and UV/visible absorption spectroscopy. The electro-catalytic properties of the fabricated PrCrO3/glassy carbon electrode (GCE) were investigated by cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS). CV and EIS profiles were useful for monitoring catalytic activity over a wide tadalafil concentration range from 7.85 µM to 1000 µM. The fabricated electrochemical sensor exhibited a linear detection range, a detection limit of 7.85 µM, excellent electro-catalytic activity, time response, real sample analysis, and sustainability over 11 consecutive cycles, and stability was observed under potentials of 5 eV, 10 eV, 20–100 eV, and 150 eV. CV results showed excellent electro-catalytic behavior because its oxygen-deficient crystal lattice produces oxygen vacancies, which assist in electron communication over the electrode surface. The electrochemical results of the perovskite/GCE suggest their future use in a comprehensive range including electrochemical biosensors, electro-catalysts, solid fuel cells, solar cells, supercapacitors, and corrosion protection layers.












Similar content being viewed by others
References
D.V.S. Rao, P. Radhakrishnanand, and V. Himabindu, Stress degradation studies on tadalafil and development of a validated stability-indicating lc assay for bulk drug and pharmaceutical dosage form. Chromatographia 67, 183 (2008).
J. Hoq, M.F. Islam, M.R. Miah, M.M. Rahman, A. Almahri, and M.A. Hasnat, Development of [I(ads)|Au(pc)] electrode to attain electrocatalytic oxidation of paracetamol: an efficient platform for quantitative analysis. J. Environ. Chem. Eng. 10, 108141 (2022).
H. Begum, M.M. Rahman, and M.A. Hasnat, Development of pt/au-co composite electrode as a highly durable and efficient electrocatalyst for methanol electro-oxidation in alkaline media. Intern. J. Energy Res. 46, 13769 (2022).
A.A. Ansari and B.D. Malhotra, Current progress in organic-inorganic hetero-nano-interfaces based electrochemical biosensors for healthcare monitoring. Coord. Chem. Rev. 452, 214282 (2022).
K. Kaviyarasu, E. Manikandan, and M. Maaza, Synthesis of CdS flower-like hierarchical microspheres as electrode material for electrochemical performance. J. Alloy. Compd. 648, 559 (2015).
A.A. Ansari, A. Kaushik, P.R. Solanki, and B.D. Malhotra, Sol–gel derived nanoporous cerium oxide film for application to cholesterol biosensor. Electrochem. Commun. 10, 1246 (2008).
A.A. Ansari, P.R. Solanki, and B.D. Malhotra, Sol-gel derived nanostructured cerium oxide film for glucose sensor. Appl. Phys. Lett. 92, 263901 (2008).
A.A. Ansari, G. Sumana, M.K. Pandey, and B.D. Malhotra, Sol–gel-derived titanium oxide-cerium oxide biocompatible nanocomposite film for urea sensor. J. Mater. Res. 24, 1667 (2009).
A.A. Ansari, P.R. Solanki, and B.D. Malhotra, Sol–gel derived nanostructured tin oxide film for glucose sensor. Sens. Lett. 7, 64 (2009).
A.A. Ansari, R. Singh, G. Sumana, and B.D. Malhotra, Sol–gel derived nano-structured zinc oxide film for sexually transmitted disease sensor. Analyst 134, 997 (2009).
A.A. Ansari, A. Kaushik, P.R. Solanki, and B.D. Malhotra, Nanostructured zinc oxide platform for mycotoxin detection. Bioelectrochemistry 77, 75 (2010).
A.A. Ansari, M. Alam, and M.A. Ali, Nanostructured CeO2: Ag platform for electrochemically sensitive detection of nitrophenol. Colloids Surf. A Physicochem. Eng. Asp 613, 126116 (2021).
A.A. Ansari and M. Alam, Electrochemical performance of the Mn-doped CeO2: nanoparticles for sensitive electrocatalysts the urea concentrations. J. Aust. Ceram. Soc. 58, 217 (2022).
P.R. Solanki, A. Kaushik, A.A. Ansari, and B.D. Malhotra, Nanostructured zinc oxide platform for cholesterol sensor. Appl. Phys. Lett. 94, 143901 (2009).
A.A. Ansari, A. Kaushik, P.R. Solanki, and B.D. Malhotra, Electrochemical cholesterol sensor based on tin oxide-chitosan nanobiocomposite film. Electroanalysis 21, 965 (2009).
P.R. Solanki, A. Kaushik, A.A. Ansari, G. Sumana, and B.D. Malhotra, Zinc oxide-chitosan nanobiocomposite for urea sensor. Appl. Phys. Lett. 93, 163903 (2008).
R. Khan, A. Kaushik, P.R. Solanki, A.A. Ansari, M.K. Pandey, and B.D. Malhotra, Zinc oxide nanoparticles-chitosan composite film for cholesterol biosensor. Anal. Chim. Acta. 616, 207 (2008).
A.A. Ansari, G. Sumana, R. Khan, and B.D. Malhotra, Polyaniline-cerium oxide nanocomposite for hydrogen peroxide sensor. J. Nanosci. Nanotechnol. 9, 4679 (2009).
A.A. Ansari, P.R. Solanki, and B.D. Malhotra, Hydrogen peroxide sensor based on horseradish peroxidase immobilized nanostructured cerium oxide film. J. Biotechnol. 142, 179 (2009).
A. Kaushik, P.R. Solanki, A.A. Ansari, G. Sumana, S. Ahmad, and B.D. Malhotra, Iron oxide-chitosan nanobiocomposite for urea sensor. Sens. Actuat. B Chem. 138, 572 (2009).
A. Kaushik, P.R. Solanki, A.A. Ansari, S. Ahmad, and B.D. Malhotra, A nanostructured cerium oxide film-based immunosensor for mycotoxin detection. Nanotechnology 20, 055105 (2009).
A. Kaushik, R. Khan, P.R. Solanki, P. Pandey, J. Alam, S. Ahmad, and B.D. Malhotra, Iron oxide nanoparticles–chitosan composite based glucose biosensor. Biosens. Bioelectron. 24, 676 (2008).
A.M. Amanulla, C.M. Magdalane, S. Saranya, R. Sundaram, and K. Kaviyarasu, Selectivity, stability and reproducibility effect of CeM-CeO2 modified PIGE electrode for photoelectrochemical behaviour of energy application. Surf. Interf. 22, 100835 (2021).
X. Fuku, N. Matinise, M. Masikini, K. Kasinathan, and M. Maaza, An electrochemically active green synthesized polycrystalline NiO/MgO catalyst: use in photo-catalytic applications. Mater. Res. Bull. 97, 457 (2018).
P.R. Solanki, A. Kaushik, A.A. Ansari, A. Tiwari, and B.D. Malhotra, Multi-walled carbon nanotubes/sol–gel-derived silica/chitosan nanobiocomposite for total cholesterol sensor. Sens. Actuat. B Chem. 137, 727 (2009).
P.R. Solanki, A. Kaushik, A.A. Ansari, G. Sumana, and B.D. Malhotra, Horse radish peroxidase immobilized polyaniline for hydrogen peroxide sensor. Polym. Adv. Technol. 22, 903 (2011).
A.A. Ansari, M.K. Nazeeruddin, and M.M. Tavakoli, Organic-inorganic upconversion nanoparticles hybrid in dye-sensitized solar cells. Coord. Chem. Rev. 436, 213805 (2021).
A.A. Ansari, V.K. Thakur, and G. Chen, Functionalized upconversion nanoparticles: new strategy towards fret-based luminescence bio-sensing. Coord. Chem. Rev. 436, 213821 (2021).
K.M. Abu-Salah, S.A. Alrokyan, M.N. Khan, and A.A. Ansari, Nanomaterials as analytical tools for genosensors. Sensors 10, 963 (2010).
A.A. Ansari, J.P. Labis, M. Alam, S.M. Ramay, N. Ahmad, and A. Mahmood, Synthesis, structural and optical properties of Mn-doped ceria nanoparticles: a promising catalytic material. Acta Metallurg. Sin. 29, 265 (2016).
A.A. Ansari, N. Ahmad, M. Alam, S.F. Adil, M.E. Assal, A. Albadri, A.M. Al-Enizi, and M. Khan, Optimization of redox and catalytic performance of LaFeO3 perovskites: synthesis and physicochemical properties. J. Electron. Mater. 48, 4351 (2019).
A.A. Ansari, N. Ahmad, M. Alam, S.F. Adil, S.M. Ramay, A. Albadri, A. Ahmad, A.M. Al-Enizi, B.F. Alrayes, M.E. Assal, and A.A. Alwarthan, Physico-chemical properties and catalytic activity of the sol–gel prepared Ce-ion doped LaMnO3 perovskites. Sci. Rep. 9, 7747 (2019).
A.A. Ansari, S.F. Adil, M. Alam, N. Ahmad, M.E. Assal, J.P. Labis, and A. Alwarthan, Catalytic performance of the Ce-doped LaCoO3 perovskite nanoparticles. Sci. Rep. 10, 15012 (2020).
P. Ciambelli, S. Cimino, S. De Rossi, L. Lisi, G. Minelli, P. Porta, and G. Russo, AFeO3(A = La, Nd, Sm) and LaFe1−xMgxO3 perovskites as methane combustion and CO oxidation catalysts: structural, redox and catalytic properties. Appl. Catal. B Environ. 29, 239 (2001).
S. Rousseau, S. Loridant, P. Delichere, A. Boreave, J.P. Deloume, and P. Vernoux, La(1−x)SrxCo1−yFeyO3 perovskites prepared by sol–gel method: characterization and relationships with catalytic properties for total oxidation of toluene. Appl. Catal. B Environ. 88, 438 (2009).
L. Zhang, Y. Zhang, J. Wei, and W. Liu, Perovskite LaFexCo1−xO3−λ deposited SiO2 catalytic membrane for deeply cleaning wastewater. Chem. Engin. J. 403, 126386 (2021).
S.S. Rathnakumar, K. Noluthando, A.J. Kulandaiswamy, J.B.B. Rayappan, K. Kasinathan, J. Kennedy, and M. Maaza, Stalling behaviour of chloride ions: a non-enzymatic electrochemical detection of α-endosulfan using cuo interface. Sens. and Actuat. B Chem. 293, 100 (2019).
M.J. Martinez-Lope, J.A. Alonso, M. Retuerto, and M.T. Fernandez-Diaz, Evolution of the crystal structure of RVO3 (R = La, Ce, Pr, Nd, Tb, Ho, Er, Tm, Yb, Lu, Y) perovskites from neutron powder diffraction. Inorg. Chem. 47, 2634 (2008).
Y.P. Chen, D.D. Wang, H.W. Qin, H. Zhang, Z.L. Zhang, G.J. Zhou, C.Y. Gao, and J.F. Hu, CO2 sensing properties and mechanism of PrFeO3 and NdFeO3 thick film sensor. J. Rare Earth 37, 80 (2019).
S.J. Li, Y.Z. Li, Y.J. Chen, L.N. Xu, Q.Y. Chen, Y. Qu, G.F. Wang, P.F. Zhu, D.S. Wang, and W.P. Qin, Enhanced visible-light photoactivities of perovskite-type LaFeO3 nanocrystals by simultaneously doping Er3+ and coupling mgo for CO2 reduction. Chemcatchem 12, 623 (2019).
R. Bornovski, L.F. Huang, E.P. Komarala, J.M. Rondinelli, and B.A. Rosen, Catalytic enhancement of co oxidation on LaFeO3 regulated by ruddlesden-popper stacking faults. ACS Appl. Mater. Inter. 11, 33850 (2019).
P.P. Wang, Y.F. He, Y. Mi, J.F. Zhu, F.L. Zhang, Y. Liu, Y. Yang, M.M. Chen, and D.W. Cao, Enhanced photoelectrochemical performance of LaFeO3 photocathode with Au buffer layer. Rsc Adv. 9, 26780 (2019).
R. Maity, M.S. Sheikh, A. Dutta, and T.P. Sinha, Visible light driven photocatalytic activity of granular Pr doped LaFeO3. J. Electron. Mater. 48, 4856 (2019).
X.T. Yin, D. Dastan, F.Y. Wu, and J. Li, Facile synthesis of SnO2/LaFeO3-XNX composite: photocatalytic activity and gas sensing performance. Nanomaterials 9, 1163 (2019).
W. Sun, W.X. Wang, D. Chen, Z.X. Cheng, T.T. Jia, and Y.X. Wang, Giant magnetoelectric coupling and two-dimensional electron gas regulated by polarization in BiFeO3/LaFeO3 heterostructures. J. Phys. Chem. C 123, 16393 (2019).
L. Ma, S.Y. Ma, Z. Qiang, X.L. Xu, Q. Chen, H.M. Yang, H. Chen, Q. Ge, Q.Z. Zeng, and B.Q. Wang, Preparation of Co-doped LaFeO3 nanofibers with enhanced acetic acid sensing properties. Mater. Lett. 200, 47 (2017).
Q. Liang, J. Jin, C.H. Liu, S. Xu, and Z.Y. Li, Constructing a novel p-n heterojunction photocatalyst LaFeO3/g-C3N4 with enhanced visible-light-driven photocatalytic activity. J. Alloy. Compd. 709, 542 (2017).
G. Iervolino, V. Vaiano, D. Sannino, L. Rizzo, and V. Palma, Enhanced photocatalytic hydrogen production from glucose aqueous matrices on Ru-doped LaFeO3. Appl. Catal. B Environ. 207, 182 (2017).
A.A. Ansari, S.F. Adil, M. Alam, N. Ahmad, M.E. Assal, J.P. Labis, and A. Alwarthan, Catalytic performance of the Ce-doped LaCoO3 perovskite nanoparticles. Sci. Rep. 10, 15012 (2020).
C. Zhang, K. Zeng, C. Wang, X. Liu, G. Wu, Z. Wang, and D. Wang, LaMnO3 perovskites via a facile nickel substitution strategy for boosting propane combustion performance. Ceram. Intern. 46, 6652 (2020).
M.A. Gabal, F. Al-Solami, Y.M. Al Angari, A.A. Ali, A.A. Al-Juaid, K.W. Huang, and M. Alsabban, Auto-combustion synthesis and characterization of perovskite-type LaFeO3 nanocrystals prepared via different routes. Ceram. Intern. 45, 16530 (2019).
A.A. Ansari, J.P. Labis, and A. Khan, Biocompatible NaYF4:Yb, Er upconversion nanoparticles: colloidal stability and optical properties. J. Saudi Chem. Soc. 25, 101390 (2021).
A.A. Ansari, A.K. Parchur, J.P. Labis, and M.A. Shar, Physiochemical characterization of highly biocompatible, and colloidal LaF3:Yb/Er upconversion nanoparticles. Photochem. Photobiol. Sci. 20, 1195 (2021).
A.A. Ansari, Comparative structural, optical, and photoluminescence studies of YF3:Pr, YF3:Pr@LaF3, and YF3:Pr@LaF3@SiO2 core–shell nanocrystals. J. Chin. Chem. Soc. 64, 440 (2017).
A.A. Ansari, Effect of surface coating on structural and photophysical properties of CePO4:Tb, nanorods. Mater. Sci. Eng. B Adv. 222, 43 (2017).
A.A. Ansari, Role of surface modification on physicochemical properties of luminescent YPO4: Tb nanorods. Colloid Surf. A 529, 286 (2017).
B.B. Kamble, M. Naikwade, K.M. Garadkar, R.B. Mane, K.K.K. Sharma, B.D. Ajalkar, and S.N. Tayade, Ionic liquid assisted synthesis of chromium oxide (Cr2O3) anoparticles and their application in glucose sensing. J. Mater. Sci. Mater. Electron. 30, 13984 (2019).
L.D. Zhang, C.M. Mo, W.L. Cai, and G. Chen, Characterizations of optical absorption in porous Al2O3·Cr2O3 nanocomposites. Nanostructured Mater. 9, 563 (1997).
Acknowledgment
The authors extend their appreciation to Researchers Supporting Project number (RSP2023R365), King Saud University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
All authors wrote and reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ansari, A.A., Khan, M.A.M. & Alam, M. Perovskite Nanoparticles and Their Use in Efficient Electro-Catalytic Oxidation of Tadalafil. J. Electron. Mater. 52, 6864–6873 (2023). https://doi.org/10.1007/s11664-023-10622-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11664-023-10622-4