Abstract
The resistive type of graphite/WO3 nanocomposite-based humidity sensor is fabricated through screen printing on a flexible polyethylene terephthalate substrate. Three different nanocomposite-based humidity sensors have been fabricated and analyzed for their humidity-sensing characteristics. The structure elucidation of the nanocomposite was carried out using x-ray diffraction, Fourier-transform infrared spectroscopy, and scanning electron microscopy. By exposing the printed humidity sensor to relative humidity ranging from 11% to 97% at room temperature, its capabilities were studied. The relative resistance, sensitivity, dynamic response, and hysteresis were determined for all three devices, and they showed maximum responses towards relative humidity changes with the highest sensitivity of ≈ 60.8% and excellent hysteresis curves (maximum change of ≈ 1%). The screen-printed flexible humidity sensor exhibited less than a 5% change in the internal electrical resistance when subjected to various bending angles.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A sensor is a device that transforms a physical signal into an electrical signal. A humidity sensor is a piece of requisite equipment that gathers information from the environment and expresses the relative humidity in the air.1,2,3,4,5 Humidity is generally acknowledged to be the proportion of water vapor in the air. Modifications in the level of humidity greatly affect the environmental conditions and, consequently, regular humidity monitoring is required. To calculate and measure the humidity levels with accuracy and precision, it is necessary to improve the sensor’s characteristics, such as response time, dynamic range, linearity, repeatability, etc.6 Humidity sensors find applications in various fields, like economic industries, food storage, medicine, agriculture, environmental health, meteorology, etc.7,8,9,10,11 Therefore, it is necessary to meet the performance requirements of humidity sensors, such as real-time skin humidity monitoring,12 respiration patterns in humans,13,14,15 non-contact sensing,16,17,18 etc.
The necessity to measure the humidity under changed environmental conditions leads to novelty in the structural pattern of the device, the explored material characteristics, and the improvement in fabrication technology. Humidity sensors possess the capability of converting fluctuations in humidity levels to electrical signals which helps in real-time humidity monitoring in each application.19,20 The real-time humidity monitoring of skin provides some useful information regarding human being’s physiological condition, growth, aging state, and wound-healing progress.21,22,23,24 Moreover, the development of humidity sensors helps to provide information about transpiration in plants during crop production via their leaf surfaces.25 Henceforth, this necessitates the development of flexible sensors by properly selecting the material’s design and structure to ensure it is appropriate for various critical applications.26,27,28 Polymers such as polydimethylsiloxane (PDMS), polyimide (PI), and polyethylene terephthalate (PET) have been reported as substrate materials for the design of flexible sensors. Another approach is to attach a sensitive material to a flexible substrate for the preparation of flexible sensors.29,30,31 To design flexible sensors with enhanced selectivity, dynamic range, recovery time, response time, etc., modifications in the properties of graphene via functionalization can be utilized.32
Due to its low cost, inherent flexibility, and increased surface area, graphite is one of the best-known nanomaterials for creating flexible devices and sensors.33,34,35,36,37,38,39 Since graphite has a high electrical conductivity and can detect single gas molecules, it has the potential to offer better sensitivity and a faster reaction time. Because of their hydrophilic nature, graphene oxide, reduced graphene oxide, and graphene, which are all derivatives of graphite, have been utilized to create wearable sensors that can measure humidity levels.40,41,42 Graphite is a kind of naturally occurring carbon that has hexagonal lattices in two dimensions (2-D) and sp2-bonded carbon. An excellent electrical conductor is a graphite structure with a sp2 nanosheet-like structure that is coupled to one another by weak van der Waal forces of attraction, and in which the electrons are delocalized due to the small energy gap between the valence and conduction bands. The different metal oxide semiconductors, such as TiO2,43 SnO2,44 WO3,45 ZnO, and In2O3,46 are promising nanostructures for detecting humidity and single gas molecules including CO2, H2S, CO, NOX, O3, etc. WO3 has been intensely studied since 1991, and is a very effective gas sensor with an oxygen-inadequate n-type semiconductor. WO3 possesses high oxidation power, a small band energy gap, a large surface area, and is capable of oxidizing H2O-O2.41,47,48,49 In recent times, the screen-printing process has emerged to create flexible humidity sensors with various design patterns and extendable output.
The primary elements of screen printing consist of a press bed, screen, paste, squeegee, and substrate, and this process offers several advantages in generating layers such as materials used in resistance, insulation, and dielectric for supercapacitor and humidity sensors.33 In this work, we have successfully reported the synthesis of a highly sensitive graphite/WO3 nanocomposite and its evaluation for humidity-sensing characteristics. The suggested flexible humidity sensor combines the benefits of being affordable, non-invasive, extremely stable, and sensitive, while retaining a great deal of potential for wearable and adaptable healthcare equipment.
Experimental
Materials and Methods
Sodium tungstate dihydrate (Na2WO4.2H2O), cetyltrimethylammonium bromide (CTAB), hydrochloric acid, diacetone alcohol C6H12O2, 98%), and graphite c, extra pure) were purchased from LOBA Chemie, as were the salts, CH3COOK, MgCl2, K2CO3, (MgNO3)2, CuCl2, NaCl, KCl, KNO3 , and K2SO4 required for the humidity sensing application. Cellulose acetate propionate C76H114O49, extra pure) was bought from Sigma-Aldrich and utilized as received. All resistance and current measurements were carried out using a Fluke 179 True Multimeter.
Preparation of Tungsten Oxide (WO3)
For the preparation of WO3 nanosheets, a simple chemical reduction precipitation approach has been used, as reported elsewhere.50 In a nutshell, CTAB (capping agent, 0.0521 g) was dissolved in an appropriate quantity of water to provide a clear solution for the WO3 synthesis. A suitable quantity of Na2WO4.2H2O was added to the reaction mixture, followed by the incorporation of dilute HCl solution dropwise, and vigorous stirring produced a pale-yellow precipitate, which was centrifuged, completely rinsed with water, and then set aside to dry. To create pure WO3 nanosheets, the obtained powder was calcined at a temperature of 500 °C.
Preparation of Graphite/ WO3 Composite
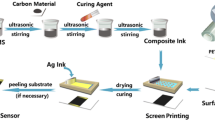
For the preparation of the graphite/WO3 (GW) nanocomposite, the synthesized WO3 powder and pure graphite were physically mixed and added to ethanol. Ultrasonication was then carried out to ensure the proper mixing of the materials and an even distribution of nanoparticles over the nanosheet, as demonstrated in Fig. 1. The prepared inks were used to print the humidity sensor using screen-printing. The graphite and synthesized WO3 were added in various ratios (1:1, 2:1, and 1:3, which were named as GW-1, GW-2, and GW-3 as shown in Table I. This simple, efficient, inexpensive, and mass-producible method was employed in synthesizing a composite intended to develop a humidity sensor. The nanocomposites were mixed with vehicle resin (mixture of solvent and binder), composed of diacetone alcohol (solvent) and cellulose acetate propionate (binder) in the ratio of 3:1. Furthermore, a few drops of triton-X100 was added to the solution followed by continuous stirring for 45 min to obtain graphite/WO3 ink, as shown in Fig. 2.
Screen-Printing of Flexible Graphite/WO3 Humidity Sensor
Screen-printing of the device was carried out using polyester-based screen fabric mesh having a mesh count of 120/cm. First, commercial silver conducting ink was used to print the electrode, which resulted in good continuity with a very low resistance of 3 \(\Omega .\) After drying the silver ink, graphite/WO3 inks with various compositions were printed. A metal connection was made to avoid harm and discontinuity during the characterizing sensor, as shown in Fig. 3. The complete overview of screen-printed humidity sensor is given in Fig. 4.
Humidity Sensing Characterization
The flexible humidity sensor characterization was carried out using a saturated solutions approach, allowing relative humidity levels to be consistently maintained. In this procedure, saturated salt solutions were made in cylindrical sealed Teflon jars to provide the necessary relative humidity (RH) values using a saturated solution,51 and, furthermore, the RH of the sealed jars was measured using a commercially available humidity sensor. The RH associated with the saturated salts employed in this experiment were LiCl (11% RH), CH3COOK (23% RH), MgCl2 (33% RH), K2CO3 (43% RH), (MgNO3)2 (54% RH), CuCl2 (62% RH), NaCl (75% RH), KCl (85% RH), KNO3 (93% RH), and K2SO4 (97% RH) at room temperature.52 Furthermore, the salt solution was kept overnight to reach the state of equilibrium (optimum relative humidity condition), and each salt solution’s humidity was cross-checked using the commercial humidity sensor. Meanwhile, the humidity sensor was connected to a Tektronix TBS 1102B oscilloscope to ensure real-time resistance change. The device was placed in air-tight Teflon containers 2 cm above the solution to analyze the change in resistance, as depicted in Fig. 5.
Results and Discussion
XRD Analysis
X-ray diffraction XRD) analysis was carried out using a Rigaku Ultima IV diffractometer, with CuK \({\upalpha }\)radiation using a monochromator at 10-kV voltage, and scanning was carried out in the range of 10–90°/min, at a scan rate of 2°/min. XRD was used to determine the phase analysis and crystallinity of the Graphite, Tungsten oxide (WO3), and their nanocomposites. As given in Fig. 6a and b, the sharp diffraction peaks observed are clear evidence for the monoclinic and orthorhombic phases of WO3.50 Figure 6a shows peaks at 23.18, 23.65, 24.33, 28.76, 33.18, 34.22, 50.14, and 55.96, which correspond to the planes at (002), (020), (200), (112), (002), (220), (040), and (100) respectively. The resultant data were further matched with the standard JCPDS cards nos. 00-024-0747 and 01-071-0131. The triplet peaks which exhibit the planes of (002), (020), and (200) with moderate intensity imply the crystallinity of the WO3. Another sharp diffraction peak at 26.4\(^\circ\) corresponds to the (002) plane of the graphite-layered structure, as per the standard JCDPS data card (00-012-0212); the peak is shown in the (002) plane in Fig. 6b. As the concentration of graphite increased, the diffraction peak intensity increased, as shown in the XRD patterns of GW-1 and GW-2. In contrast with GW-3, the composition is increased by three-fold with respect to graphite, which leads to an increase in the triplet characteristic peak for WO3 nanoparticles. The crystalline size of WO3 was calculated using the Debye–Scherrer equation53,54:
where D represents the crystalline particle size, K stands for the Scherrer constant, \(\lambda\) denotes the x-ray wavelength, and \(\beta\) represents the full width at half-maximum of the diffraction peak. The calculated average crystalline size of the WO3 nanoparticles is 236 nm.
FT-IR Analysis
The study of the molecular bond structure of the synthesized WO3 nanoparticles was examined using Fourier-transform infrared spectroscopy (FT-IR) within 400–4000 cm−1. The spectrum of the synthesized WO3 nanoparticles is shown in Fig. 7, which shows the FT-IR spectrum of pure WO3 crystalline nanoparticles with vibrational bands at 3592, 807, and 684. The stretching and bending vibrations of O-H molecules in the absorbed water molecules from the surroundings are attributed to the detected IR bands at 3592 cm−1 and 1530 cm−1. The stretching and bridging vibrations of W-Oint-W in the WO3 nanoparticles are also shown by large IR bands at 807 cm−1 and 684 cm−1.55,56
SEM Analysis
From the XRD analysis,, it was found that the tungsten oxide is present in two phases, the monoclinic and the orthorhombic. The SEM images of tungsten oxide and its nanocomposites also correlate with the dual phase nature of the nanoparticles of tungsten oxide, and, for the determination of the sizes and morphologies of the synthesized WO3 and its nanocomposite, are given in Fig. 8a–e. The SEM image of WO3 exhibits a granular nanoparticle morphology, as shown in Fig. 8a, which reveals the distinct nanoparticles with remarkable continuity of the particles, which in turn reduces the internal resistance of the device. Figure 8b–d demonstrates the composites of pristine graphite and WO3 nanoparticles, which can be easily differentiated based on their structural morphologies. The accumulated WO3 nanoparticles on the graphitic sheet are circled in Fig. 8b. As the concentration of the graphite increases, it causes agglomeration and restacking of the nanosheets, as seen in Fig. 8c, whereas the optimum distribution of the WO3 nanoparticles over the graphite nanosheet can be easily visualized in Fig. 8d. The screen-printed silver (current collector) is even and finely printed over the PET substrate while maintaining good continuity and having exceptionally low resistances of \(\sim\) 3 \({\Omega }\) , which is highly advantageous when it comes to a flexible sensor to ensure its continuity while twisting and bending, as shown in Fig. 8e.
Sensing Mechanism
The sensing process is based on the change in the resistance of composite materials caused by the water molecules which were adsorbed on the surfaces, and the humidity can be effectively determined by measuring the resistance change across the sensor.57 Tungsten oxide is considered a promising sensing material for humidity-sensing applications because of its merits, such as being sensitive in a wide relative humidity range, showing a fast response toward variations in relative humidity, and providing physical and chemical stability. The sensing phenomenon for tungsten oxide nanoparticles can be understood by considering that electrons get trapped on the surface defects such as ionized oxygen vacancies, and then get released when molecules of water are adsorbed onto the defect sites.58 The resistive response of sensing material depends upon the concentration of the moisture content which can be driven by two factors, i.e., chemisorption and physisorption of water molecules adhering to the surface of the sensor material. Under a low moisture content, only chemisorption takes place, which implies the adsorption of the first water molecule layer over the sensing material surface, which strongly adheres to the oxide surface at around 30–40% RH concentration.59 The present oxygen absorbs in the form of ions like O−, O2−, and O2−2:
As the relative humidity (RH%) concentration increases, it leads to the formation of a physio-absorbed layer over the chemisorbed and the process continues over the numerous layers. However, all the physio-absorbed layers can be removed by either raising the temperature or lowering the RH% concentration because they are volatile (unstable) in nature, causing the device’s resistance to increase:
At this phase of the process, H3O+ ions with a significant number of H+ protons develop and begin to serve as a charge carrier, also known as the Grotthuss mechanism.60,61,62 These charge carrier (H+) protons start hopping from one to another water molecule, through the simultaneous making and breaking of hydrogen bonds with an adjacent water molecule, and release an excess of protons, which leads to a dramatic decrease in the resistance. Furthermore, water molecules begin condensing into the underlying porous structural arrangement within the bulk of the sensor material, and a new carrier channel for ion migration is produced:
Humidity Sensor Characterization
The variation of resistance with respect to the RH of nanocomposites was carried out at room temperature (25 °C) in RH% conditions is shown in Fig. 9a and b. The resistance of the GW-2 (168–211 \( {\Omega }\)) device is comparatively less than the GW-1 (313–353 \( {\Omega }){ }\) device, while the GW-3 device has a resistance varying in the range of 4.2–6.47 k\(\Omega\) due to an increase in the content of WO3 in composite GW-3. To account for the sensing performance of the device, various parameters such as sensitivity, hysteresis, and dynamic response awerrere carried out, namely relative resistance with respect to resistance, as shown in Fig. 9a. It can be seen in Fig. 10 that the GW-3 composite shows a better performance and remarkable improvement during the determination of the real sensitivity compared to the other composites. This may be a huge change in resistance in accordance with the RH, and due to enhanced physical parameters like bonding between WO3 and graphite, and water adsorption capacity. The sensitivity of the flexible humidity sensor was calculated using:
where S (%) represents the sensitivity, Rlowest symbolizes the device’s resistance at lower humidity (11%), and Rcurrent represents resistance at the current humidity, The sensitivity of the GW-3 composite shows an essentially linear rise with the increase in humidity, which is evident in the case of the high RH% values, as can be seen in Fig. 10. As the RH% increases, the resistance and sensitivity of the sensor shows a direct increasing trend.
For the estimation of hysteresis in the humidity sensor, the resistance is measured throughout the device in various RH conditions: RH% 11–97% response and from 97% to 11% recovery. The resultant output differs from the initial response at the same RH value, which is nothing but defined as hysteresis. During the hysteresis, the difference is observed which depends upon the rate of absorption and desorption of water vapor on the sensing layer of the device during the sensing of humidity. In GW-1 and GW-2, the sensors showed high hysteresis compared to GW-3, which is nearly superimposed over each other at various RH (%) ranges and shows the excellent reliability of the sensors. The absorption and desorption curves of the humidity sensor are shown in Fig. 11a–c. The dynamic response curve of the humidity sensor is measured concerning the resistance change for time (s), as shown in Fig. 12a–c. For the GW-3, Fig. 12c has a steady change in resistance compared to the other nanocomposites. As the concentration of graphite is greater in these composites, this leads to a decrease in the response change towards the RH condition.
Flexibility Analysis
The printed humidity sensor (GW-3) was subjected to different bending angles \(0^\circ ,\;30^\circ ,\;60^\circ ,\;90^\circ ,\;{\text{and 120}}^\circ\) using a customized device, as reported elsewhere.63 As demonstrated in Fig. 13a, the absolute resistance of the screen-printed GW-3 at each angle was measured using a digital probe multimeter (Fluke 179). There is a very minimal change observed during the electrical resistance of the sensor at a different bending angle, as shown in Fig. 13b. Table II compares the fabrication and sensing methods between previously reported work and the present work.
Conclusions
We have successfully synthesized and characterized highly sensitive graphite/WO3 nanocomposites as screen-printed humidity sensors on a PET substrate. The analysis of a flexible humidity sensor was carried out to measure the relative change, sensitivity, hysteresis, and dynamic pattern of responses. The results obtained reveal that the devices (GW-1, GW-2, and GW-3) showed quite good results towards resistance but, as the graphite concentration increases in GW-3, the resistance decreases and respective change is much smaller. In these comparative studies of all the sensors (GW-1, GW-2, and GW-3), GW-3 exhibited an excellent performance with a high rate of change of resistance and a sensitivity of about ≈ 60.8%, with excellent hysteresis curves (maximum change ≈ 1%). GW-3 also exhibits steady adsorption and desorption patterns. Moreover, the screen printing makes it a better sensor than a conventional solid-state sensor.
References
T.Q. Trung, S. Ramasundaram, B.-U. Hwang, and N.-E. Lee, An all-elastomeric transparent and stretchable temperature sensor for body-attachable wearable electronics. Adv. Mater. 28, 502 (2016).
P. Zhu, Y. Liu, Z. Fang, Y. Kuang, Y. Zhang, C. Peng, and G. Chen, Flexible and highly sensitive humidity sensor based on cellulose nanofibers and carbon nanotube composite film. Langmuir 35, 4834 (2019).
T. Li, L. Li, H. Sun, Y. Xu, X. Wang, H. Luo, Z. Liu, and T. Zhang, Porous ionic membrane based flexible humidity sensor and its multifunctional applications. Adv. Sci. 4, 1600404 (2017).
G. Zhou, J.-H. Byun, Y. Oh, B.-M. Jung, H.-J. Cha, D.-G. Seong, M.-K. Um, S. Hyun, and T.-W. Chou, Highly sensitive wearable textile-based humidity sensor made of high-strength, single-walled carbon nanotube/poly(vinyl alcohol) filaments. ACS Appl. Mater. Interfaces 9, 4788 (2017).
X. Fu, J.K. Wang, A.C. Ramírez-Pérez, C. Choong, and G. Lisak, Flexible conducting polymer-based cellulose substrates for on-skin applications. Mater. Sci. Eng. C 108, 110392 (2020).
T. Delipinar, A. Shafique, M.S. Gohar, and M.K. Yapici, Fabrication and materials integration of flexible humidity sensors for emerging applications. ACS Omega 6, 8744 (2021).
J. Choi, Y. Chen, R. Abbel, I. Visagie, and K. Parker, Flexible humidity sensors for wireless monitoring based on electrospun sulfonated polyether ether ketone (SPEEK) nanofibres. Sens. Actuators B Chem. 324, 128704 (2020).
Z. Duan, Y. Jiang, Q. Zhao, Q. Huang, S. Wang, Y. Zhang, Y. Wu, B. Liu, Y. Zhen, and H. Tai, Daily writing carbon ink: novel application on humidity sensor with wide detection range, low detection limit and high detection resolution. Sens. Actuators B Chem. 339, 129884 (2021).
X. Wang, Z. Xiong, Z. Liu, and T. Zhang, Exfoliation at the liquid/air interface to assemble reduced graphene oxide ultrathin films for a flexible noncontact sensing device. Adv. Mater. 27, 1370 (2015).
F. Zhao, Y. Zhao, H. Cheng, and L. Qu, A graphene fibriform responsor for sensing heat, humidity, and mechanical changes. Angew. Chem. Int. Ed. 54, 14951 (2015).
P. Yasaei, A. Behranginia, T. Foroozan, M. Asadi, K. Kim, F. Khalili-Araghi, and A. Salehi-Khojin, Stable and selective humidity sensing using stacked black phosphorus flakes. ACS Nano 9, 9898 (2015).
Z. Zhen, Z. Li, X. Zhao, Y. Zhong, L. Zhang, Q. Chen, T. Yang, and H. Zhu, Formation of uniform water microdroplets on wrinkled graphene for ultrafast humidity sensing. Small 14, 1703848 (2018).
Y. Pang, J. Jian, T. Tu, Z. Yang, J. Ling, Y. Li, and T.L. Ren, Wearable humidity sensor based on porous graphene network for respiration monitoring. Biosens. Bioelectron. 116, 123 (2018).
Z. Wu, J. Yang, X. Sun, Y. Wu, L. Wang, G. Meng, and Y. He, An excellent impedance-type humidity sensor based on halide perovskite CsPbBr3 nanoparticles for human respiration monitoring. Sens. Actuators B Chem. 337, 129772 (2021).
J. Dai, H. Zhao, X. Lin, S. Liu, Y. Liu, X. Liu, T. Fei, and T. Zhang, Ultrafast response polyelectrolyte humidity sensor for respiration monitoring. ACS Appl. Mater. Interfaces 11, 6483 (2019).
H. Tai, Z. Duan, Y. Wang, S. Wang, and Y. Jiang, Paper-based sensors for gas, humidity, and strain detections: a review. ACS Appl. Mater. Interfaces 12, 31037 (2020).
S. Mondal, S.J. Kim, and C.-G. Choi, Honeycomb-like MoS2 nanotube array-based wearable sensors for noninvasive detection of human skin moisture. ACS Appl. Mater. Interfaces 12, 17029 (2020).
F.J. Romero, A. Rivadeneyra, M. Becherer, D.P. Morales, and N. Rodríguez, Fabrication and characterization of humidity sensors based on graphene oxide–PEDOT: PSS composites on a flexible substrate. Micromachines 11, 148 (2020).
M. Parrilla, T. Guinovart, J. Ferré, P. Blondeau, and F.J. Andrade, A wearable paper-based sweat sensor for human perspiration monitoring. Adv. Healthc. Mater. 8, 1900342 (2019).
R. Alrammouz, J. Podlecki, A. Vena, R. Garcia, P. Abboud, R. Habchi, and B. Sorli, Highly porous and flexible capacitive humidity sensor based on self-assembled graphene oxide sheets on a paper substrate. Sens. Actuators B. Chem. 298, 126892 (2019).
T.Q. Trung, L.T. Duy, S. Ramasundaram, and N. Lee, Transparent, stretchable, and rapid-response humidity sensor for body-attachable wearable. Electronics 4, 1 (2016).
S.L. Kobal, L. Trento, S. Baharami, K. Tolstrup, T.Z. Naqvi, B. Cercek, and R.J. Siegel, Comparison of effectiveness of hand-carried ultrasound to bedside cardiovascular physical examination. Am. J. Cardiol. 96(7), 1002 (2005).
F.H. Wilhelm and W.T. Roth, The somatic symptom paradox in DSM-IV anxiety disorders: suggestions for a clinical focus in psychophysiology. Biol. Psychol. 57, 105 (2001).
C. Ashley, D. Burton, Y.B. Sverrisdottir, M. Sander, D.K. Mckenzie, and V.G. Macefield, Firing probability and mean firing rates of human muscle vasoconstrictor neurones are elevated during chronic asphyxia. J. Psychol. 4, 701 (2010).
S. Yin, H. Ibrahim, P.S. Schnable, M.J. Castellano, L. Dong, and A. Field-Deployable, Wearable leaf sensor for continuous monitoring of vapor-pressure deficit. Adv. Mater. Technol. 6, 2001246 (2021).
L. Lan, X. Le, H. Dong, J. Xie, Y. Ying, and J. Ping, Biosensors and bioelectronics one-step and large-scale fabrication of flexible and wearable humidity sensor based on laser-induced graphene for real-time tracking of plant transpiration at bio-interface. Biosens. Bioelectron. 165, 112360 (2020).
J. Yang, R. Shi, Z. Lou, R. Chai, K. Jiang, and G. Shen, Flexible smart noncontact control systems with ultrasensitive humidity sensors. Small 15(38), 1902801 (2019).
L. Liu, W. Chen, H. Zhang, Q. Wang, F. Guan, and Z. Yu, Flexible and multifunctional silk textiles with biomimetic leaf-like MXene/silver nanowire nanostructures for electromagnetic interference shielding, humidity monitoring, and self‐derived hydrophobicity. Adv. Funt. Mater. 29, 1905197 (2019).
Y. Guo, Z. Gao, X. Wang, L. Sun, and X. Yan, A highly stretchable humidity sensor based on spandex covered yarns and nanostructured polyaniline. RSC Adv. 8, 1078 (2017).
X. Wang, W. Zhao, S. Al-Abdul-Wahid, Y. Lu, T. Cheng, J.J. Madsen, and L. Ye, Trifluorinated keto-enol tautomeric switch in probing domain rotation of a G protein-coupled receptor. Bioconjugate Chem. 32(1), 99 (2020).
X. Guan, Y. Yu, Z. Hou, K. Wu, H. Zhao, S. Liu, and T. Zhang, A flexible humidity sensor based on self-supported polymer film. Sens. Actuators B Chem. 358, 131438 (2022).
S. Iravani, Bacteria in nanoparticle synthesis: current status and future prospects. Int. Sch. Res. Notices 2014, 359316 (2014).
R. Soukup, A. Hamacek, and J. Reboun, Advanced Screen Printing for the Fabrication of Organic Humidity Sensors, in 2012 4th Electronic system-integration technology conference (IEEE, 2012), pp. 1–5
T. Nakajima, T. Nakamura, and T. Tsuchiya, Flexible humidity sensors composed of graphite-like carbon micro-pinecone arrays. RSC Adv. 6, 95342 (2016).
V. Balakrishnan, T. Dinh, A.R.M. Foisal, T. Nguyen, H.P. Phan, D.V. Dao, and N.T. Nguyen, Paper-based electronics using graphite and silver nanoparticles for respiration monitoring. IEEE Sens. J. 19, 11784 (2019).
C. Steffens, A. Manzoli, R.T. Paschoalin, L. Tiggemann, J. Steffens, E. Teixeira, and P.S. de Paula Herrmann, Tracing paper substrate used for development of interdigitated graphite electrode and its application as humidity sensor. Synth. Met. 183, 36 (2013).
S.E. Bourdo, B.A. Warford, and T. Viswanathan, Electrical and thermal properties of graphite/polyaniline composites. J. Solid State Chem. 196, 309 (2012).
L. Wang, D. Wang, G. Zhu, J. Li, and F. Pan, Thermoelectric properties of conducting polyaniline/graphite composites. Mater. Lett. 65, 1086 (2011).
M. Fujikane, K. Kurosaki, H. Muta, and S. Yamanaka, Thermoelectric properties of Ag8GeTe6. J. Alloys Compd. 396, 280–282 (2005).
S. Yu, H. Zhang, C. Chen, J. Zhang, and P. Li, Preparation and mechanism investigation of highly sensitive humidity sensor based on two-dimensional porous gold/graphite carbon nitride nanoflake. Sens. Actuators B Chem. 307, 127679 (2020).
S.-J. Choi, F. Fuchs, R. Demadrille, B. Grévin, B.-H. Jang, S.-J. Lee, J.-H. Lee, H.L. Tuller, and I.-D. Kim, Fast responding exhaled-breath sensors using WO3 hemitubes functionalized by graphene-based electronic sensitizers for diagnosis of diseases. ACS Appl. Mater. Interfaces 6, 9061 (2014).
F. Ullah-Khan, S. Mahmood, Z. Ahmad, T. Mahmood, and Z.A. Nizami, Graphene oxide synthesis by facile method and its characterization. Open J. Chem. 2, 11 (2019).
R.J. Wu, Y.L. Sun, C.C. Lin, H.W. Chen, and M. Chavali, Composite of TiO2 nanowires and Nafion as humidity sensor materialSens. Actuators B Chem. 115, 198 (2006).
E. Comini, G. Faglia, G. Sberveglieri, Z. Pan, and Z.L. Wang, Stable and highly sensitive gas sensors based on semiconducting oxide nanobelts. Appl. Phys. Lett. 81, 1869 (2002).
Q. Qi, T. Zhang, Q. Yu, R. Wang, Y. Zeng, L. Liu, and H. Yang, Properties of humidity sensing ZnO nanorods-base sensor fabricated by screen-printing Sens. Actuators B Chem. 133, 638 (2008).
J. Huang and Q. Wan, Gas sensors based on semiconducting metal oxide one-dimensional nanostructures. Sensors 9, 9903 (2009).
M.V. Arularasu, R. Sundaram, C. Maria-Magdalane, K. Kanimozhi, K. Kaviyarasu, F.T. Thema, D. Letsholathebe, G.T. Mola, and M. Maaza, Synthesis, humidity sensing, photocatalytic and antimicrobial properties of thin film nanoporous PbWO4-WO3 nanocomposites. J. Nanostruct. 7, 47 (2017).
A. Dinesh, M.S. Anantha, M.S. Santosh, M.G. Priya, K. Venkatesh, K.S. Yogesh-Kumar, M.S. Raghu, and H.B. Muralidhara, Improved performance of iron-based redox flow batteries using WO3 nanoparticles decorated graphite felt electrode. Ceram. Int. 47, 10250 (2021).
A. Staerz, C. Berthold, T. Russ, S. Wicker, U. Weimar, and N. Barsan, The oxidizing effect of humidity on WO3 based sensors, sensors actuators. B Chem. 237, 54 (2016).
H.U. Khan, M. Tariq, M. Shah, S. Ullah, A.R. Ahsan, A. Rahim, J. Iqbal, R. Pasricha, and I. Ismail, Designing and development of polyvinylpyrrolidone-tungsten trioxide (PVP-WO3) nanocomposite conducting film for highly sensitive, stable, and room temperature humidity sensing. Mater. Sci. Semicond. Process. 134, 106053 (2021).
E. Ouda, N. Yousf, M. Morsy, and E.-S.M. Duraia, Flexible humidity sensor based on light-scribed graphene oxide. J. Mater. Sci. Mater. Electron. 33, 18241 (2022).
S. Manjunatha, T. Machappa, Y.T. Ravikiran, B. Chethan, and A. Sunilkumar, Polyaniline based stable humidity sensor operable at room temperature. Phys. B Condens. Matter 561, 170–178 (2019).
N.A. Pithawala and B. Jain, Halloysite nanotubes and applications: a review. J. Adv. Sci. Res. 1, 19 (2012).
U. Holzwarth and N. Gibson, The Scherrer equation versus the Debye–Scherrer equation. Nat. Nanotechnol. 6, 534 (2011).
J. Jayachandiran, M. Arivanandhan, O. Padmaraj, R. Jayavel, and D. Nedumaran, Investigation on ozone-sensing characteristics of surface sensitive hybrid RGO/WO3 nanocomposite films at ambient temperature. Adv. Compos. Hybrid Mater. 3, 16 (2020).
S. Prabhu, S. Manikumar, L. Cindrella, and O.J. Kwon, Charge transfer and intrinsic electronic properties of RGO-WO3 nanostructures for efficient photoelectrochemical and photocatalytic applications. Mater. Sci. Semicond. Process. 74, 136 (2018).
A. Nag, A. Mitra, and S.C. Mukhopadhyay, Graphene and its sensor-based applications: a review. Sens. Actuators A Phys. 270, 177 (2018).
N.K. Pandey, K. Tiwari, and A. Roy, Characterization and humidity sensing application of ZnO-TiO2 nanocomposite. Adv. Mater. Res. 304, 48 (2011).
G.M.G. Silva, V.N.S. Leão, M.F.G. Pereira, P.M. Faia, and E.S. Araújo, Zn2+-doped TiO2:WO3 films prepared by electrospinning and sintering: microstructural characterization and electrical signature to moisture sensing. Ceramics 4, 576 (2021).
N. Agmon, The Grotthuss mechanism. Chem. Phys. Lett. 244, 456 (1995).
B. Chethan, H.G. Raj-Prakash, Y.T. Ravikiran, S.C. Vijaya-Kumari, S. Manjunatha, and S. Thomas, Humidity sensing performance of hybrid nanorods of Polyaniline–Yttrium oxide composite prepared by mechanical mixing method. Talanta 215, 120906 (2020).
M. Ahmadipour, M.F. Ain, and Z.A. Ahmad, Fabrication of resistance type humidity sensor based on CaCu3Ti4O12 thick film. Meas. J. Int. Meas. Confed. 94, 902 (2016).
R. Nayak, P. Shetty, S.M.A. Rao, and K.M. Rao, Formulation of new screen printable PANI and PANI/graphite based inks: printing and characterization of flexible thermoelectric generators. Energy 238, 121680 (2022).
N.K. Pandey, A. Roy, K. Tiwari, A. Mishra, A. Rai, S. Jayaswal, M. Rashmi, and A. Govindan, Humidity sensor based on synthesized pure WO3 and WO3-SnO2 nanocomposite, in 2012 1st International symposium on physics and technology of sensors (ISPTS-1) (IEEE, 2012), pp. 129–132.
T.Y. Ling, S.H. Pu, S.J. Fishlock, Y. Han, J.D. Reynolds, J.W. McBride, and H.M.H. Chong, Sensing performance of nanocrystalline graphite-based humidity sensors. IEEE Sens. J. 19, 5421 (2019).
M.S. Pawar, P.K. Bankar, M.A. More, and D.J. Late, Ultra-thin V2O5 nanosheet based humidity sensor, photodetector and its enhanced field emission properties. RSC Adv. 5, 88796 (2015).
M. Parthibavarman, M. Karthik, and S. Prabhakaran, Facile and one step synthesis of WO3 nanorods and nanosheets as an efficient photocatalyst and humidity sensing material. Vaccum 155, 224 (2018).
Funding
Open access funding provided by Manipal Academy of Higher Education, Manipal. Ms. Shazneen shiraj is grateful to MAHE for the support of seed money grant (seed money id 00000566).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Saquib, M., Shiraj, S., Nayak, R. et al. Synthesis and Fabrication of Graphite/WO3 Nanocomposite-Based Screen-Printed Flexible Humidity Sensor. J. Electron. Mater. 52, 4226–4238 (2023). https://doi.org/10.1007/s11664-023-10404-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11664-023-10404-y