Abstract
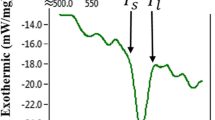
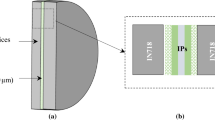
The Ag-41.0 at.%Sb alloy has a melting point at 485°C and is a suitable braze alloy for mid-temperature joining processes. Co and Ni are frequently used as barrier layer materials. The Co/(Ag-41.0 at.%Sb)1−xCox and Ni/(Ag-41.0 at.%Sb)1−xNix interfacial reactions at 550°C are studied. Two phases, CoSb and CoSb3, are formed in all Co/(Ag-41.0 at.%Sb)1−xCox couples for which the value of x is 0, 0.01, 0.03 and 0.05. CoSb is a thin layer and the reaction region with CoSb3 phase is a thick region in which granular CoSb3 is mixed with the liquid phase. Two phases, Ni5Sb2 and NiSb, are formed in Ni/(Ag-41.0 at.%Sb)1−xNix couples for which the value of x is 0, 0.01 and 0.05. Both Ni5Sb2/NiSb and NiSb/liquid interfaces are planar. The reaction rates in the Co/Ag-Sb and Ni/Ag-Sb couples are high, and the reaction rates in the Ni/Ag-Sb couples are even higher. Doping Co decreases the reaction rates in the Co/(Ag-41.0 at.%Sb)1−xCox couples, but doping Ni has no noticeable effect upon the reaction rates for the Ni/(Ag-41.0 at.%Sb)1−xNix couples.
Similar content being viewed by others
References
R.J. Klein Wassink, Soldering in Electronics, 2nd ed. (British Isles: Electrochemical Publications Limited, 1989).
K.N. Subramanian, Lead-Free Solders: Materials Reliability for Electronics (West Sussex: Wiley, 2012).
M. Schwartz, Brazing: For the Engineering Technologist (London: Chapman & Hall, 1995).
S.-W. Chen, Mater. Chem. Phys. 33, 271 (1993).
S.-W. Chen, L.-C. Chen, and J.-C. Wang, J. Alloys Compd. 694, 93 (2017).
C.-S. Oh, J.-H. Shim, B.-J. Lee, and G.N. Lee, J. Alloys Compd. 238, 155 (1996).
W.-J. Zhu, H.-S. Liu, J.-S. Wang, H.-Q. Dong, and Z.-P. Jin, J. Alloys Compd. 481, 503 (2009).
Y. Zhang, C. Li, Z. Di, and T. Geng, Calphad 32, 56 (2008).
T. Shimozaki, K.S. Kim, T. Iwata, T. Okino, and C.G. Lee, Mater. Trans. 43, 2609 (2002).
F.J.J. van Loo, Prog. Solid State Chem. 20, 47 (1990).
Acknowledgments
The authors acknowledge the financial support of Ministry of Science and Technology (Grant MOST 103-2221-E-007-021-MY3) and High Entropy Materials Center in National Tsing Hua University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chen, Sw., Peng, YS. & Chen, Lc. Co/(Ag-41.0 at.%Sb)1−xCox and Ni/(Ag-41.0 at.%Sb)1−xNix Interfacial Reactions. J. Electron. Mater. 48, 5743–5756 (2019). https://doi.org/10.1007/s11664-019-07385-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11664-019-07385-2