Abstract
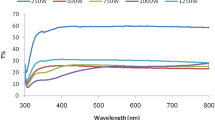
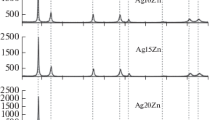
We describe a green process for the generation of silver oxide (Ag2O) nanoparticles from silver material through an electrochemical spark (discharge) process. The annealing operation was carried out on the produced nanoparticles to observe changes in the particle morphology and different properties. Ag2O nanoparticles were characterized by x-ray diffraction analysis, Fourier-transform infrared spectroscopy, ultraviolet–visible spectroscopy, field emission scanning electron microscopy, energy-dispersive x-ray (EDX) spectroscopy and high-resolution transmission electron microscopy. With the rise in annealing temperature, the average crystal size of Ag2O nanoparticles was increased proportionally and the shape was also changed. Plate-type structures were attained with high annealing temperatures. The EDX result confirmed the presence of silver and oxygen atoms. The band gap of the nanoparticle samples, which were produced by direct current and pulsating direct current, was noted to be 1.6 eV and 1.9 eV, respectively.
Similar content being viewed by others
References
J. Schoonman, Solid State Ion. 135, 5 (2000).
A. Ito, M. Shinkai, H. Honda, and T. Kobayashi, J. Biosci. Bioeng. 100, 1 (2005).
A. Yoshida and N. Toshima, J. Electron. Mater. 43, 1492 (2014).
S. Guo and E. Wang, Anal. Chim. Acta 598, 181 (2007).
J. Beltran-Huarac, J. Palomino, O. Resto, J. Wang, W.M. Jadwisienczak, B.R. Weiner, and G. Morell, RSC Adv. 4, 38103 (2014).
W. Wang, Q. Zhao, J. Dong, and J. Li, Int. J. Hydrog. Energy 36, 7374 (2011).
E. Sanli, B.Z. Uysal, and M.L. Aksu, Int. J. Hydrog. Energy 33, 2097 (2008).
V.V. Petrov, T.N. Nazarova, A.N. Korolev, and N.F. Kopilova, Sens. Actuator B Chem. 133, 291 (2008).
Y. Ida, S. Watase, T. Shinagawa, M. Watanabe, M. Chigane, M. Inaba, A. Tasaka, and M. Izaki, Chem. Mater. 20, 1254 (2008).
W.-X. Li, C. Stampfl, and M. Scheffler, Phys. Rev. Lett. 90, 256102 (2003).
Y.-H. Wang and H.-Y. Gu, Microchim. Acta 164, 41 (2009).
R.J. Chimentao, I. Kirm, F. Medina, X. Rodriguez, Y. Cesteros, P. Salagre, and J.E. Sueiras, Chem. Commun. 7, 846 (2004).
C. Fang, A.V. Ellis, and N.H. Voelcker, Electrochim. Acta 59, 346 (2012).
S. Jradi, L. Balan, X.H. Zeng, J. Plain, D.J. Lougnot, P. Royer, R. Bachelot, S. Akil, O. Soppera, and L. Vidal, Nanotechnology 21, 95605 (2010).
K.K. Caswell, C.M. Bender, and C.J. Murphy, Nano Lett. 3, 667 (2003).
D.C. Tien, C.Y. Liao, J.C. Huang, K.H. Tseng, J.K. Lung, T.T. Tsung, W.S. Kao, T.H. Tsai, T.W. Cheng, and B.S. Yu, Rev. Adv. Mater. Sci 18, 750 (2008).
C.-H. Lo, T.-T. Tsung, and H.-M. Lin, J. Alloys Compd. 434, 659 (2007).
D.-C. Tien, K.-H. Tseng, C.-Y. Liao, and T.-T. Tsung, J. Alloys Compd. 473, 298 (2009).
L. Godson, B. Raja, D.M. Lal, and S. Wongwises, Exp. Heat Transf. 23, 332 (2010).
K.-H. Tseng, Y.-C. Chen, and J.-J. Shyue, J. Nanopart. Res. 13, 1865 (2011).
A.A. Ashkarran, Appl. Phys. A 107, 401 (2012).
M.A. Etman, Nanosci. Nanotechnol. 3, 56 (2013).
M. Stein, D. Kiesler, and F.E. Kruis, J. Nanopart. Res. 15, 1400 (2013).
J.-P. Borra, N. Jidenko, J. Hou, and A. Weber, J. Aerosol Sci. 79, 109 (2015).
A.A. Ashkarran, Curr. Appl. Phys. 10, 1442 (2010).
M. Miranzadeh and M.Z. Kassaee, Chem. Eng. J. 257, 105 (2014).
M.M. Rahman, S.B. Khan, A.M. Asiri, and A.G. Al-Sehemi, Electrochim. Acta 112, 422 (2013).
J. Fang, P.M. Leufke, R. Kruk, D. Wang, T. Scherer, and H. Hahn, Nano Today 5, 175 (2010).
P. Kumar, P.K. Singh, M. Hussain, and A. Kumar Das, Adv. Sci. Lett. 22, 3 (2016).
P.K. Singh, P. Kumar, M. Hussain, A.K. Das, and G.C. Nayak, Bull. Mater. Sci. 39, 469 (2016).
R. Wüthrich and A. Allagui, Electrochim. Acta 55, 8189 (2010).
A. Hickling and M.D. Ingram, J. Electroanal. Chem. 8, 65 (1959).
A. Hickling and M.D. Ingram, Trans. Faraday Soc. 60, 783 (1964).
F. Fang, J. Kennedy, E. Manikandan, J. Futter, and A. Markwitz, Chem. Phys. Lett. 521, 86 (2012).
S.U. Ilyas, R. Pendyala, and N. Marneni, Chem. Eng. Technol. 37, 2011 (2014).
I. Haas, S. Shanmugam, and A. Gedanken, J. Phys. Chem. B 110, 16947 (2006).
W. He, X. Duan, L. Zhu, and J. Cent, S. Univ. Technol. 16, 708 (2009).
S. Deshpande, S. Patil, S.V.N.T. Kuchibhatla, and S. Seal, Appl. Phys. Lett. 87, 133113 (2005).
B.D. Cullity and S.R. Stock, X-Ray Diffraction, 2nd ed. (Reading: Addison-Wesley, 1978), pp. 102–111.
S. Rehman, A. Mumtaz, and S.K. Hasanain, J. Nanopart. Res. 13, 2497 (2011).
C.C. Baker, A. Pradhan, and S.I. Shah, Encyclopedia of Nanoscience and Nanotechnology (Valencia: American Scientific Publishers, 2004), pp. 449–473.
E. Esmaeili, M. Salavati-Niasari, F. Mohandes, F. Davar, and H. Seyghalkar, Chem. Eng. J. 170, 278 (2011).
N. Goswami and D.K. Sharma, Phys. E Low. Dimens. Syst. Nanostruct. 42, 1675 (2010).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Singh, P.K., Bishwakarma, H., Shubham et al. Study of Annealing Effects on Ag2O Nanoparticles Generated by Electrochemical Spark Process. J. Electron. Mater. 46, 5715–5727 (2017). https://doi.org/10.1007/s11664-017-5614-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11664-017-5614-6