Abstract
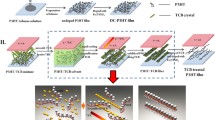
Poly(3-hexylthiophene) (P3HT) films were prepared by using raw P3HT powders with different molecular configurations through solution processing and doped with ferric salt of triflimide anions [Fe(TFSI)3], and their thermoelectric (TE) properties were studied. It was found that P3HT with highly regular molecular configuration formed highly ordered chain arrangements in P3HT film (denoted rr-P3HT film), while P3HT with irregular molecular configuration formed random chain arrangements in P3HT film (denoted ra-P3HT film). The ordered chain arrangement in rr-P3HT not only improved the charge carrier mobility but also contributed to produce more carriers, thereby remarkably improving the electrical conductivity. The electrical conductivity of rr-P3HT film was up to 96.1 S/cm, more than two orders of magnitude higher than that of ra-P3HT film. Consequently, the power factor of rr-P3HT film reached 17.10 μW/(m K2), about one order of magnitude higher than that of ra-P3HT film and among the best values for pure P3HT TE materials. This study suggests that P3HT with highly regular configuration contributes to form highly ordered molecular chain arrangements, resulting in improved TE properties.
Similar content being viewed by others
References
Q. Zhang, Y. Sun, W. Xu, and D. Zhu, Adv. Mater. 26, 6829 (2014).
Y. Chen, Y. Zhao, and Z. Liang, Energy Environ. Sci. 8, 401–422 (2015).
M. Chang, J. Lee, N. Klenhenz, B. Fu, and E. Reichmanis, Adv. Funct. Mater. 24, 4457 (2014).
D.M. DeLongchamp, R.J. Kline, Y. Jung, D.S. Germack, E.K. Lin, A.J. Moad, L.J. Richter, M.F. Toney, M. Heeney, and I. McCulloch, ACS Nano. 3, 780 (2009).
Y. He, H. Chen, J. Hou, and Y. Li, J. Am. Chem. Soc. 132, 1377 (2010).
J. Sun, M.L. Yeh, B.J. Jung, B. Zhang, J. Feser, A. Majumdar, and H.E. Katz, Macromolecules 43, 2897 (2010).
Y. Xuan, X. Liu, S. Desbief, P. Leclere, M. Fahlman, R. Lazzaroni, M. Berggren, J. Cornil, D. Emin, and X. Crispin, Phys. Rev. B 82, 115454 (2010).
M. He, J. Ge, Z. Lin, and F. Qiu, Energy Environ. Sci. 5, 8351 (2012).
Q. Zhang, Y. Sun, W. Xu, and D. Zhu, Energy Environ. Sci. 5, 9639–9644 (2012).
K. Yoshino, Jpn. J. Appl. Phys. 29, L995 (1990).
K. See, J. Feser, and R. Segalman, Nano. Lett. 10, 4664 (2010).
N. Coats, S. Yee, B. McCulloch, R. Segalman, and J. Urban, Adv. Mater. 25, 1629 (2013).
Q. Yao, Q. Wang, L. Wang, Y. Wang, J. Sun, H. Zeng, Z. Jin, X. Huang, and L. Chen, J. Mater. Chem. A 2 8, 2634 (2014).
A.B. Kaiser and V. Skakalova, Chem. Soc. Rev. 40, 3786 (2011).
A.B. Kaiser, Adv. Mater. 13, 927 (2001).
Q. Yao, L. Chen, W. Zhang, S. Liufu, and X. Chen, ACS Nano. 4, 2445 (2010).
Q. Wang, Q. Yao, J. Chang, and L. Chen, J. Mater. Chem. 22, 17612 (2012).
H.Y. Mao, B. Xu, and S. Holdcroft, Macromolecules 26, 1163 (1993).
B. Xu and S. Holdcroft, Macromolecules 26, 4457 (1993).
R.M. Souto Maior, K. Hinkelmann, H. Eckert, and F. Wudl, Macromolecules 23, 1268 (1990).
T.-A. Chen and R.D. Rieke, J. Am. Chem. Soc. 114, 10087 (1992).
T. Stocker, A. Kohler, and R.J. Moos, J. Polym. Sci. B 50, 976–983 (2012).
J.L. Bredas and G.B. Street, Acc. Chem. Res. 18, 309 (1985).
D. Ofer, R.M. Crooks, and M.S. Wrighton, J. Am. Chem. Soc. 112, 7869 (1990).
Acknowledgements
This work was supported by the National Basic Research Program of China (973 Program, 2013CB632506), the Key Research Program of the Chinese Academy of Sciences (Grant No. KGZD-EW-T06), the National Natural Science Foundation of China (Grant No. 51102268), and the China Postdoctoral Science Foundation (Grant No. 2013M541554).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Qu, S., Yao, Q., Shi, W. et al. The Influence of Molecular Configuration on the Thermoelectrical Properties of Poly(3-hexylthiophene). J. Electron. Mater. 45, 1389–1396 (2016). https://doi.org/10.1007/s11664-015-4045-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11664-015-4045-5