Abstract
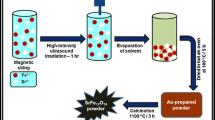
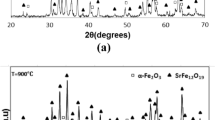
Strontium hexaferrite (SrFe12O19, SrM) suitable for high-performance permanent magnet applications was synthesized by salt-assisted ultrasonic spray pyrolysis (SA-USP) and subsequent calcination. To control the particle size, the intermediate phase of SrM was collected by SA-USP and various sizes of SrM were obtained by calcining the as-prepared sample at temperatures ranging from 800°C to 1050°C. The synthesized SrM was magnetically aligned by using an external magnetic field to improve remanence. The synthesized particles were of nano- to submicron scale and nonagglomerated. The magnetic properties and squareness of the material depended on the particle size and distribution. Additionally, the NaCl added during synthesis facilitated the formation of nonagglomerated particles, while enhancing and controlling particle growth. The optimum magnetic properties were achieved at calcination temperature of 1000°C, resulting in coercivity of 5646 Oe, saturation magnetization of 73.3 emu/g, and remanence of 59.1 emu/g (80.6% of M s).
Similar content being viewed by others
References
R.C. Pullar, Prog. Mater. Sci. 57, 1191 (2012).
K.S. Martirosyan, C. Dannangoda, E. Galstyan, and D. Litvinov, J. Appl. Phys. 111, 094311 (2012).
S.E. Jacobo, C. Herme, and P.G. Bercoff, J. Alloys Compd. 495, 513 (2010).
J.S. Jiang, J.E. Pearson, Z.Y. Liu, B. Kabius, S. Trasobares, D.J. Miller, S.D. Bader, D.R. Lee, D. Haskel, G. Srajer, and J.P. Liu, Appl. Phys. Lett. 85, 5293 (2004).
J.M.D. Coey, Scr. Mater. 67, 524 (2012).
P. Tenaud, A. Morel, F. Kools, J.M. Le Breton, and L. Lechevallier, J. Alloys Compd. 370, 331 (2004).
A. Goldman, Modern Ferrite Technology (New York: Springer, 2006), p. 227.
M. Cernea, S.-G. Sandu, C. Galassi, R. Radu, and V. Kuncser, J. Alloys Compd. 561, 121 (2013).
D. Chen, Y. Liu, Y. Li, K. Yang, and H. Zhang, J. Magn. Magn. Mater. 337–338, 65 (2013).
P.E. Kazin, L.A. Trusov, D.D. Zaitsev, Y.D. Tretyakov, and M. Jansen, J. Magn. Magn. Mater. 320, 1068 (2008).
B.K. Rai, S.R. Mishra, V.V. Nguyen, and J.P. Liu, J. Alloys Compd. 550, 198 (2013).
T. Kikuchi, T. Nakamura, T. Yamasaki, M. Nakanishi, T. Fujii, J. Takada, and Y. Ikeda, J. Magn. Magn. Mater. 322, 2381 (2010).
S. Ounnunkad, Solid State Commun. 138, 472 (2006).
F.J. Berry, J.F. Marco, C.B. Ponton, and K.R. Whittle, J.␣Mater. Sci. Lett. 20, 431 (2001).
A. Drmota, M. Drofenik, and A. Žnidaršič, Ceram. Int. 38, 973 (2012).
E. Kiani, A.H. Rozatian, and M. Yousefi, J. Mater. Sci. Mater. Electron. 24, 2485 (2013).
L. Junliang, Z. Yanwei, G. Cuijing, Z. Wei, and Y. Xiaowei, J. Eur. Ceram. Soc. 30, 993 (2010).
P. Xu, X. Han, and M. Wang, J. Phys. Chem. C 111, 5866 (2007).
M. Radwan, M.M. Rashad, and M.M. Hessien, J. Mater. Process. Technol. 181, 106 (2007).
D. Lisjak and M. Drofenik, J. Eur. Ceram. Soc. 26, 3681 (2006).
Ashima, S. Sanghi, and Reetu, J. Alloys Compd. 513, 436 (2012).
D.H. Han, J.P. Wang, and H.L. Luo, J. Magn. Magn. Mater. 136, 176 (1994).
S.V. Ketov, Y.D. Yagodkin, and V.P. Menushenkov, J. Alloys Compd. 509, 1065 (2011).
I. Nedkov, A. Petkov, and V. Cheparin, J. Magn. Magn. Mater. 83, 430 (1990).
J. Dufour, E. López-Vidriero, C. Negro, R. Latorre, E.M. AlcalÁ, F. López-Mateos, and A. Formoso, Chem. Eng. Commun. 167, 227 (1998).
H.M. Lee, S.-Y. Bae, J.-H. Yu, and Y.-J. Kim, J. Am. Ceram. Soc. 91, 2856 (2008).
M.H. Kim, D.S. Jung, Y.C. Kang, and J.H. Choi, Ceram. Int. 35, 1933 (2009).
S. Dursun, R. Topkaya, N. Akdoğan, and S. Alkoy, Ceram. Int. 38, 3801 (2012).
R.H. Arendt, J. Solid State Chem. 8, 339 (1973).
B. Xia, I.W. Lenggoro, and K. Okuyama, Adv. Mater. 13, 1579 (2001).
G.-H. An, T.-Y. Hwang, J. Kim, J. Kim, N. Kang, S. Kim, Y.-M. Choi, and Y.-H. Choa, J. Alloys Compd. 583, 145 (2014).
C. Wang, L. Li, J. Zhou, X. Qi, Z. Yue, and X. Wang, J. Magn. Magn. Mater. 257, 100 (2003).
X. Liu, P. Hernández-Gómez, K. Huang, S. Zhou, Y. Wang, X. Cai, H. Sun, and B. Ma, J. Magn. Magn. Mater. 305, 524 (2006).
Z.F. Zi, Y.P. Sun, X.B. Zhu, Z.R. Yang, J.M. Dai, and W.H. Song, J. Magn. Magn. Mater. 320, 2746 (2008).
G. Cao, Nanostructures and Nanomaterials, (Imperial College Press, 2004).
M.M. Hessien, M.M. Rashad, and K. El-Barawy, J. Magn. Magn. Mater. 320, 336 (2008).
B.D. Cullity and C.D. Graham, Introduction to Magnetic Materials, (Piscataway, NJ: Wiley, 2009).
Z.-B. Guo, W.-P. Ding, W. Zhong, J.-R. Zhang, and Y.-W. Du, J. Magn. Magn. Mater. 175, 333 (1997).
Acknowledgements
We acknowledge a National Research Foundation of Korea (NRF) Grant funded by the Korea government (MSIP) (No. 2008-0061891) and support from the Pioneer Research Center Program through the National Research Foundation of Korea (NRF-2012-0001262) funded by the Ministry of Science, ICT, and Future Planning.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
An, GH., Hwang, TY., Choa, YH. et al. Synthesis of Size-Controlled SrFe12O19 Using Modified Spray Pyrolysis–Calcination Method and Their Magnetic Properties. J. Electron. Mater. 43, 3574–3581 (2014). https://doi.org/10.1007/s11664-014-3228-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11664-014-3228-9