Abstract
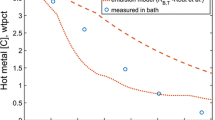
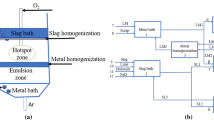
Examining the kinetics involved in the Argon Oxygen Decarburization (AOD) process, especially in the hotspot and emulsion zones within distinct reactors, can offer a deeper understanding of the refining mechanism in stainless-steelmaking. A predictive dynamic model has been formulated to estimate the effects of different refining processes, encompassing decarburization, desiliconization, demanganization, and chromium removal. The model includes a sub-model for heat loss calculation. The FactSage™ software, along with its macro programming capability, was utilized to incorporate thermochemical and kinetic information into the model. The model forecasts that the predominant chromium removal occurs within the hotspot zone, while carbon, silicon, and manganese removals occur in both the hotspot and emulsion zones. The predictions regarding the transient compositions of steel and slag, as well as the temperature of the steel bath, align with the plant data (Average of five heats), showcasing consistency.







Similar content being viewed by others
References
https://www.worldstainless.org. Accessed 17 June 2023.
M. Ersson and A. Tilliander: Steel Res. Int., 2018, vol. 89, p. 1700108.
S. Chanouian, B. Ahlin, A. Tilliander, and M. Ersson: Steel Res. Int., 2022, vol. 93, pp. 1–11.
T. Ohno and T. Nishida: Tetsu-to Hagane, 1977, vol. 63, pp. 2094–99.
S. Asai and J. Szekely: Metall. Trans, 1974, vol. 5, pp. 651–57.
J. Szekely and S. Asai: Metall. Trans, 1974, vol. 5, pp. 1573–80.
V.V. Visuri, M. Jarvinen, P. Sulasalmi, E.P. Heikkinen, J. Savolainen, and T. Fabritius: ISIJ Int., 2013, vol. 53, pp. 603–12.
V.V. Visuri, M. Jarvinen, P. Sulasalmi, E.P. Heikkinen, J. Savolainen, and T. Fabritius: ISIJ Int., 2013, vol. 53, pp. 613–21.
V.V Visuri, R Mattila, P Kupari, T.A Fabritius, Comparative study on refractory wear associated with fluxes for AOD slags. In Proceedings of the 7th International Congress on Science and Technology of Steelmaking, Venice 13–15 June 2018.
Y. Kang, Y.H. Kim, and H.-S. Sohn: Met. Mater. Int., 2015, vol. 21, pp. 118–25.
K. Ashok, P. Sankar, and K. Maruthupandian: J. Metall. Mater. Sci., 2017, vol. 59, pp. 173–80.
R. Fruehan: Ironmak. Steelmak., 1976, vol. 3, pp. 153–58.
K. Miyamoto, K. Kato, and T. Yuki: Tetsu -to- Hagane, 2002, vol. 88, pp. 838–44.
S. Yokoyama, M. Takeda, K. Ito, and M. Kawakami: Tetsu-to Hagane, 1992, vol. 78, pp. 223–30.
T. Nakasuga, K. Nakashima, and K. Mori: ISIJ Int., 2004, vol. 44, pp. 665–72.
T. Nakasuga, H. Sun, K. Nakashima, and K. Mori: ISIJ Int., 2001, vol. 41, pp. 937–44.
T. Nakasuga, H. Sun, K. Nakashima and K. Mori: Proceeding of the 2nd International Conference on processing materials for properties, vol. 86, TMS, Warrendale, PA, 2000, pp. 553.
E. Shibata, S. Egawa, and T. Nakamura: ISIJ Int., 2002, vol. 42, pp. 609–13.
K. Taoka, M. Tada, H. Nomura, and H. Baba: Tetsu-to-Hagane, 1990, vol. 76, pp. 1863–870.
M. Jarvinen, S. Pisila, A. Karna, T. Fabritius, T. Ikaheimonen, and P. Kupari: Steel Res. Int., 2011, vol. 82, pp. 638–49.
M. Jarvinen, S. Pisila, A. Karna, T. Fabritius, T. Ikaheimonen, and P. Kupari: Steel Res. Int., 2011, vol. 82, pp. 650–57.
J.-H. Wei and D.-P. Zhu: Metall. Mater. Trans. B, 2002, vol. 33, pp. 111–19.
J.H. Wei and D.P. Zhu: Metall. Mater. Trans. B, 2002, vol. 33, pp. 121–27.
M. Gornerup and P. Sjoberg: Ironmak. Steelmak., 1999, vol. 26, pp. 58–63.
Wei Chi-ho and A. Mitchell, Application of mathematical and physical models in the iron and steel industry, In Proceeding 3rd Process Technology Conference, March 28–31, 1982, ISS, Pittsburgh, PA, vol. 3, pp. 232–254.
A. Mitchell, F.R. Carmona, and C. Wei: Iron Steelmak, 1982, vol. 3, pp. 37–41.
J. Wei and A. Mitchell: Chin. J. Met. Sci. Technol., 1986, vol. 2, pp. 11–23.
J. Wei and A. Mitchell: Acta Metall. Sin., 1989, vol. 23, pp. 126–34.
W. Jihe: Chin. J. Met. Sci. Technol., 1989, vol. 5, pp. 32–46.
E. Shibata, H. Sun, and K. Mori: Metall. Mater. Trans. B, 1999, vol. 30, pp. 279–86.
M. Gornerup and A.K. Lahiri: Ironmak. Steelmak., 1998, vol. 25, pp. 317–22.
B. Deo and S. Kumar: Adv Mat Res, 2013, vol. 794, pp. 50–62.
J. Riipi, T. Fabritius, E.-P. Heikkinen, P. Kupari, and A. Karna: ISIJ Int., 2009, vol. 49, pp. 1468–476.
E.P. Heikkinen, V.V. Visuri and T. Fabritius, Proceeding of the 8th European Oxygen Steelmaking Conference, Associazione Italiana di Metallurgia, Taranto 2018.
P. Ternstedt, G. Runnsjo, A. Tilliander, J. Janis, N.A.I. Andersson, and P.G. Jonsson: Metals, 2020, vol. 10, p. 308.
E.P. Heikkinen, T.M.J. Fabritius, T.M.T. Kokkonen, and J.J. Harkki: Steel Res. Int., 2004, vol. 75, pp. 800–806.
P. Ternstedt, R. Gyllenram, J. Bengtsson and P.G. Jonsson, Proceeding of the 4th International Conference on Modelling and Simulation of Metallurgical Processes in Steelmaking, Stahlinstitut VDEh, Düsseldorf 2011.
E. Wimmer, D. Kahrimanovic, K. Pastucha, B. Voraberger, and G. Wimmer: Berg Huettenmaenn Monatsh, 2020, vol. 165, pp. 3–10.
H.J. Odenthal, U. Thiedemann, U. Falkenreck, and J. Schlueter: Metall. Trans. B, 2010, vol. 41B, pp. 396–413.
C. Wuppermann, N. Giesselmann, A. Ruckert, H. Pfeifer, H.J. Odenthal, and E. Hovestadt: ISIJ Int., 2012, vol. 52, pp. 1817–823.
C. Wuppermann, A. Ruckert, H. Pfeifer, and H.J. Odenthal: ISIJ Int., 2013, vol. 53, pp. 441–49.
W. Wei, J. Gustavsson, P.B. Samuelsson, R. Gyllenram, A. Tilliander, and P.G. Jonsson: Ironmak. Steelmak., 2022, vol. 49, pp. 70–82.
A. Illiander, L.T.I. Jonsson, and P.G. Jonsson: Steel Res. Int., 2014, vol. 85, pp. 376–87.
P. Amuelsson, P. Ternstedt, A. Tilliander, A. Appell, and P.G. Jonsson: Ironmak. Steelmak., 2017, vol. 45, pp. 1–7.
S. Patra, J. Nayak, L.K. Singhal, and S. Pal: Steel Res. Int., 2017, vol. 88, p. 1600271.
P. Ternstedt, P. Ni, N. Lundqvist, A. Tilliander, and P.G. Jonsson: Ironmak. Steelmak., 2018, vol. 45, pp. 944–50.
E.P. Heikkinen, V.V Visuri, T. Fabritius, In Proceedings of the 8th European Oxygen Steelmaking Conference, Taranto 10–12 October 2018.
A. Rafiei, G.A. Irons, and K.S. Coley: Metall. Mater. Trans. B, 2021, vol. 52, pp. 2509–25.
P. Mason, A.N. Grundy, R. Rettig, L. Kjellqvist, J. Jeppsson, J. Bratberg, 11th In International Symposium on High-Temperature Metallurgical Processing. The Minerals, Metals & Materials Series. Springer, Cham. 2020.
A.N. Grundy, M. Powell, R. Rettig, L. Kjellqvist, J. Jeppsson, A. Jansson, J. Bratberg, A Kinetic and Thermodynamic Description of the Steel Making Process using Thermo-Calc and the CALPHAD Database TCOX. https://thermocalc.com/products/add-on-modules/process-metallurgy-module/. Accessed 1 October 2023.
www.factsage.com. Accessed 10 August 2022.
P. Singha and A.K. Shukla: Metals, 2022, vol. 12, p. 638.
Acknowledgments
The author wishes to express gratitude to J Mohon Rao fQ2or providing valuable comments on the AOD plant.
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendices
Appendix 1
Initial, A = B = C = 1
Now model was run according to Figure 2, corresponding to the input parameters outlined in Table I.
The convergence criteria obtained from plant trials are applied both before and at the end of the reduction stage of the process
Now, to meet converge criteria A, B, and C values changes
Best fitted with plant data when A = 0.09, B = 0.3, and C = 0.6 only. I.e., we finally got the Eqs. [24–26]
Appendix 2
Heat Loss Model
The heat of dissolution of C, Si, Mn, Cr, and Ni is 2.11, − 4.67, 0, 0.40, and 0 KJ/kg
The specific heat of Fe, C, Si, Mn, and Cr is 0.945, 2.278,3.064,1.258, and 1.687 KJ/kg
The heat of the formation (1600 °C) of SiO2, MnO, Cr2O3 and FeO are 37.5, 7.1, 11.6 and 6.05 MJ/kg
The sensible heat of steel, CO, CO2, and slag is 1.413, 1.86. 1.86 and 2.915 MJ/kg
Heat of formation of CaO–SiO2 is 4.5 MJ/kg of Si
By solving the above two Eqs. [A4] and [A5], we get heat input = 2207.3 MJ and heat output = 1891.7 MJ.
Therefore, heat losses = 315.6 MJ/THM
Total heat losses of the system = 142 × 227.89 = 44815.2 MJ
Heat loss per time step of process = 1120.38 MJ
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Singha, P. Refining Contribution at Hotspot and Emulsion Zones of Argon Oxygen Decarburization: Fundamental Analysis Based upon the FactSage-Macro Program Approach. Metall Mater Trans B (2024). https://doi.org/10.1007/s11663-024-03084-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11663-024-03084-4