Abstract
A mathematical model for the decarburization coupling with the fluid flow in the RH refining process was established. The decarburization reaction at three sites was considered, including the surface of injection lifting gas bubbles, the gas–liquid interface in the vacuum chamber and the interior of the molten steel bath. The effect of the flow pattern on the transfer of carbon and oxygen in the molten steel was obtained. The decarburization inside the molten steel bath in the vacuum chamber contributed the most, accounting for 55.9 pct of the total decarburization. The gas–liquid interface in the vacuum chamber and the surface of bubbles injected from the up-leg snorkel contributed approximately 32.5 and 11.6 pct, respectively. The oxygen blowing through the top of the vacuum chamber promoted the decarburization of RH refining whenever the oxygen was injected. When the oxygen was injected at the beginning of the decarburization process, the contribution of the decarburization at the top surface of the molten steel in the vacuum chamber to the total decarburization increased from 11.6 to 28.3 pct.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The RH (Ruhrstahl–Hereaeus) refining process is usually used in the production of ultra-low carbon aluminum killed steel, supplemented by oxygen blowing operations, to achieve the purpose of deep decarburization and reduce the carbon content in molten steel to less than 20 ppm. The improvement of decarburization efficiency in the RH refining process is of great significance to the production of ultra-low carbon steel.
Based on the basic theory of metallurgical physical chemistry, through the combination with computer technology, there have been many reports on the coupling phenomenon of fluid flow and physicochemical reaction in the metallurgical process.[1,2] In the actual production process, thermodynamics can give whether the decarburization reaction can occur or not, while the reaction rate of the decarburization reaction is affected by kinetic factors, involving the mass transfer of carbon and oxygen in molten steel. As an important step in the production of ultra-low carbon steel, the mechanism and simulation of the decarburization process of RH have been concerned[3,4,5] and a large number of reports have been made on the decarburization reaction in various stages of the steelmaking process.[6,7,8,9,10,11,12]
In recent decades, there are researches on the flow and mixing phenomenon in the RH refining process, which includes both numerical modeling and experimental works. However, the numerical simulation application on the chemical reactions is relatively race. In the study of decarburization reaction in the RH refining process, the existing models mainly discuss the thermodynamic and kinetic mechanism of decarburization.[13,14,15,16,17] Only few of them involve the coupling calculation of three-dimensional molten steel flow and carbon-oxygen mass transfer process.[5,18,19]
In the current work, based on the steady flow field of the RH refining process obtained from the previous numerical simulation, the decarburization reaction of the RH refining process is simulated by user-defined scalar (UDS) equation. The variation of carbon and oxygen content in the molten steel was calculated, and the contribution of three reaction regions to decarburization reaction is analyzed.
Multiphase Fluid Flow During RH Refining Process
Modeling Descriptions
Firstly, the fluid flow during the RH refining process was calculated using the k–ε model. Based on the steady-state fluid flow, the decarburization reaction model considering three sites, bubbles surface, interior and the surface of the molten steel in the vacuum chamber was established.
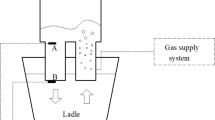
The volume of fraction (VOF) model is adopted to simulate the multiphase flow, which is used to calculate the phase interface between molten steel and argon gas. The discrete phase model (DPM) is used to calculate the trajectory of argon bubbles and the interaction between argon bubbles and molten steel. The turbulent model is the k–ε model. The mesh and boundary conditions are shown in Figure 1. The total number of cells used in the calculation is about 480,000. The material properties and the dimensions of RH treatment used in the calculation are listed in Tables I and II. The detailed model parameters could be found in the authors’ previous study.[20,21,22,23]
The assumptions concerned in the current calculation are as follows,
-
(a)
The fluids, molten steel, and gas, are Newtonian, viscous, and incompressible.
-
(b)
To simplify the model, the slag is neglected, considering there is almost no slag in the vacuum chamber.
-
(c)
Bubbles are assumed to be spherical and their deformation, coalescence, and breakup are neglected.
-
(d)
The fluid flow in the RH reactor is assumed to be an isothermal process. The temperature variation and its effect on the fluid flow are neglected.
-
(e)
The decarburization process calculation is based on a steady-state flow field.
Numerical Simulation Procedures
The simulation processing was performed using the ANSYS Fluent 17.0 commercial software, based on the finite volume method. The PISO algorithm was carried out for the pressure-velocity coupling. The spatial discretization for momentum used the second-order upwind method, and the transient formulation was second-order implicit. The convergence criterion for the continuity of momentum was 10−5. The calculation time step was 0.001 seconds.
Fluid Flow and Bubbles Distribution
Figure 2 shows the distribution of three-dimensional velocity streamlines and turbulent kinetic dissipation rate of RH ladle and vacuum chamber. The steel level in the vacuum chamber is the iso-surface with a 0.7 volume fraction of molten steel. It can be seen that the velocity of molten steel in the snorkels is fast, and the fluid forms eddy in the ladle and vacuum chamber. When the argon gas is blown into the vacuum chamber, the free surface of the molten steel in the vacuum chamber is blown up to a certain height.
The bubble surface is another vital site for decarburization reaction during the RH refining process. Figure 3 shows the spatial distribution of movement velocity and residence time of argon bubbles during the argon injection from the up-leg snorkel to the surface of molten steel in the vacuum chamber. The average residence time of bubbles from blowing to moving to the surface of molten steel in the vacuum chamber is about 1.5 seconds.
Decarburization of the Molten Steel During RH Refining Process
Formulation of the Decarburization
The basic equation of decarburization reaction of molten steel[24] was:
The equilibrium constant at 1873 K can be calculated by,
where, PCO is the partial pressure of CO gas, Pa; \(P^{\Theta }\) is the standard atmospheric pressure, Pa; [pct C] and [Pct O] are the mass concentration of dissolved carbon and dissolved oxygen at reaction interface respectively, wt pct; f[C] and f[O] are the activity coefficients of carbon and oxygen in the molten steel respectively, and the formulas are as follows:
where, \(e_{i}^{j}\) is the activity interaction coefficient. In this study, the effects of carbon, oxygen, aluminum, silicon and manganese on the activity coefficients of carbon and oxygen in molten steel are considered. In the calculation of decarburization reaction, the content of carbon and oxygen in molten steel will change with time. It is considered that the composition of other elements remains unchanged. According to the changes of carbon and oxygen in molten steel, the activities of carbon and oxygen are calculated using the current carbon and oxygen content. But the influence of other components on the activities of carbon and oxygen is excluded. The activity interaction coefficient used in the calculation is shown in Table III.
According to the molecular weight, for every 3 kg of carbon consumed, 4 kg of oxygen is consumed, as follows.
where, \(- \frac{{{\text{d}}\left[ {{\text{pct}}\;{\text{C}}} \right]}}{{{\text{d}}t}}\) and \(- \frac{{{\text{d}}\left[ {{\text{pct}}\;{\text{O}}} \right]}}{{{\text{d}}t}}\) are the reaction rates of [C] and [O] in the molten steel respectively, wt pct/s; KC and KO are the apparent rate constants, s−1; [pct C]e and [pct O]e are the equilibrium [C] and [O] contents, wt pct.
The above equation assumes that the chemical reaction is not the controlling step, and the relationship between equilibrium [C] and [O] concentration could be derived as follows[19]:
Three Decarburization Reaction Sites
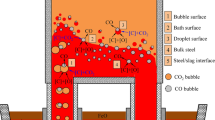
Decarburization reaction in the RH refining process mainly occurs on three sites: inside of molten pool (interface between CO bubble and molten steel), surface of molten pool in vacuum chamber and surface of argon bubble in circulating gas, as shown in Figure 4.
Interior of the molten steel
When the equilibrium partial pressure of CO, \(P_{{{\text{CO}}}}^{{\text{e}}}\), corresponding to the [C] and [O] content in the molten steel is greater than the sum of the static pressure Plocal and the pressure Pcritical, the decarburization reaction can occur,
where, σgl is the interfacial tension between generated CO bubbles and the molten steel, N/m; \(r_{{{\text{CO}}}}\) is the radius of the generated CO bubble, m. In previous studies,[14,27] when the value of Pcritical is 0.02 atm, the calculated values are in good agreement with the experimental values.
According to the proportion of [C] and [O], the restrictive step is determined. The critical [C] and [O] ratio calculation formula[16] is as follows:
where, MC and MO are the molar mass of carbon and oxygen, 12 and 16 g/mol, respectively; Q is the volume circulation flow of the molten steel, m3/s; which is calculated according to the longitudinal velocity and the cross-sectional area of the down-leg snorkel, \(Q = A_{{{\text{down}}}} \cdot \overline{u}_{{{\text{down}}}} = \pi \times 0.325^{2} \times 0.71 = 0.235\;{\text{m}}^{3} {\text{/s}}\); AinteriorkC,interior and AinteriorkO,interior are volumetric mass transfer coefficients of carbon and oxygen in the molten steel, m3/s; kC,interior and kO,interior are the mass transfer coefficients of carbon and oxygen in the molten steel, m/s. The volumetric mass transfer coefficients were related to the flow field, which was calculated during the simulation process and changed with calculation time.
When the mass transfer of [C] is the restrictive step:
When the mass transfer of [O] is the restrictive step:
where, Vinterior is the volume of the molten steel in which internal decarburization occurs, m3. The mass transfer coefficients of carbon and oxygen in the molten steel are calculated by the formula[5,16]:
Free surface of the molten steel in the vacuum chamber
In order to meet the composition requirements of ultra-low carbon steel, the decarburization reaction was accelerated by blowing oxygen[11] in the vacuum chamber during the RH refining process. After oxygen is blown into the steel liquid surface of the vacuum chamber, oxygen dissolves into the steel liquid. The Reaction Eq. [28] is as follows:
However, the existence of carbon in molten steel will limit the dissolution of oxygen in the molten steel. Under a certain temperature and pressure, the product of carbon and oxygen is constant. Therefore, it is necessary to calculate the dissolved oxygen content of molten steel according to the carbon content on the surface of molten steel. When the carbon content in the molten steel is high, oxygen is difficult to dissolve into the molten steel, but reacts with the carbon in the molten steel. The Eq. [24], which is the sum of the above Reaction Eqs. [1] and [21].
When there is oxygen blowing in the vacuum chamber, assuming that the controlling step of the carbon–oxygen reaction at the gas–liquid interface in the vacuum chamber is the mass transfer of carbon, the equilibrium carbon content, [pct C]e, at the phase interface in the vacuum chamber is calculated according to formula [26], and then the equilibrium oxygen content, [pct O]e, at the phase interface is calculated according to formula [3]. The apparent decarburization reaction rate and oxygen dissolution rate at the phase interface of the vacuum chamber are as follows:
where, kC,surface is the mass transfer coefficient of carbon on the free surface of the molten steel in the vacuum chamber, and the value in the calculation is 0.005 m/s[17]; Asurface is the area of the gas-liquid interface in the vacuum chamber, m2; Vsurface is the volume of the molten steel occurring surface decarburization reaction, m3.
A certain oxygen blowing period is set in the model, the reaction on the surface of molten steel in the vacuum chamber is Eq. [24] when oxygen blowing, and Eq. [1] when no oxygen blowing. The equilibrium carbon content at the interface calculated by the two methods is different, and there is no oxygen dissolution reaction on the surface of molten steel in the vacuum chamber when no oxygen blowing. No matter whether oxygen blowing or not, the reaction in the molten steel and on the surface of argon bubbles is the Reaction Eq. [1].
It should be pointed out that oxygen blowing into the top of the vacuum chamber will affect the flow of molten steel in the vacuum chamber, but this study ignored the effect of oxygen blowing on the flow field of the molten steel, and only considered the dissolution and diffusion of oxygen on the surface of the molten steel in the vacuum chamber.
Argon bubbles surface
The apparent rate constant of decarburization reaction on the surface of argon bubble, KC,bubble, is expressed as:
where, Abubble is the contact area between argon bubble and the molten steel, m2; DC is the diffusion coefficient of [C] in the molten steel, which is 2.24 × 10−8 m2/s[29,30]; te is the contact time, s; db is the diameter of argon bubble, m; uR is the relative velocity between the argon bubble and the molten steel, m/s.
where, DO is the diffusion coefficient of [O] in the molten steel, which is 1.26 × 10−8 m2/s.[29,30]
According to the proportion of [C] and [O], the restrictive step is determined, in which the critical [C] and [O] ratio calculation formula[16] is as follows:
When the mass transfer of [C] is a limiting step, KO,bubble is calculated according to the KC,bubble calculated by formula [32]:
When the mass transfer of [O] is a limiting step, KC,bubble is calculated according to the KO,bubble calculated by formula [36]:
In practice, the CO partial pressure of the decarburization reaction on the surface of argon bubbles is the ratio of the molar amount of CO generated to the total molar amount of argon bubbles:
where PCO,Bubble is the partial pressure of CO generated on the surface of argon bubble, Pa; PBubble,local is the static pressure of argon bubble, Pa; \(n_{{{\text{CO}}}}^{b}\) is the molar amount of CO generated on the surface of argon bubble, mol; \(n_{{{\text{Ar}}}}^{b}\) is the molar amount of argon in the argon bubble, mol. Considering the current work is based on a steady-state (shown in Figures 2 and 3), and bubbles residence time is about 1.5 seconds, which is short. Thus, the PCO, bubble was assumed to be zero, meaning the bubble is a vacuum for the formed CO gas produced by carbon and oxygen reaction.
To summarized, some key parameters are listed in Table IV.
Decarburization Model Coupling with Fluid Flow
According to the reaction conditions in different reaction regions, the reaction rates corresponding to different positions are calculated firstly, and then the decarburization reaction is coupled with fluid flow, and finally the spatial distribution of carbon and oxygen concentration in molten steel is predicted.
The variation of carbon and oxygen concentration in the molten steel during RH refining is solved by the concentration equation of carbon and oxygen:
where [C] and [O] are the mass percent contents of carbon and oxygen in molten steel, wt pct. S[C] and S[O] are the reaction source terms of the mass concentration changes of carbon and oxygen caused by decarburization reaction and the mass concentration changes of oxygen caused by the oxygen blowing process:
Based on the obtained three-dimensional flow field, the mechanism and transport model of decarburization reaction are added to the calculation by User-defined Functions (UDF), and the concentration fields of carbon and oxygen in three-dimensional flow space are solved.
Calculation Conditions and Process
The initial carbon and oxygen contents in the molten steel are 300 and 600 ppm, respectively. The distribution of carbon and oxygen content is uniform in the molten steel. The boundary condition on the walls of the RH reactor is the fluxes of the carbon and oxygen contents are zero, meaning the wall has no effect on the carbon and oxygen variation. The computational iteration process is shown in Figure 5. For each cell, the input parameters are the local carbon content, local oxygen content, and fluid features, and then the reaction zone was judged, corresponding mechanism was employed to calculate kC, kO, PCO, KC, KO, S[C], and S[O], sequentially. The obtained decarburization rate was coupled with the flow field, based on it, the carbon and oxygen content was updated in each iteration time step, and the new carbon and oxygen content was used as the input parameter of the next time step. The calculation time step is 0.1 seconds, and the total calculation time is 1200 seconds.
Results of the Decarburization Model Calculation
Model Validation
Limited by the extremely difficult sampling on each reaction zones in the vacuum chamber, the verification process comes from the comparison of carbon content between the sampling in the ladle and a monitoring point in the calculation, as shown in Figure 6. The monitor is located between the snorkels and the ladle wall in the RH ladle at 300 mm below the steel liquid level, corresponding to the sampling position of the industrial test. The specific position is shown in Figure 6(a). The calculation condition is that the argon gas flow rate is 3000 NL/min, oxygen blowing operation is carried out in 0 to 3 minutes, and the oxygen blowing flow rate is 1200 Nm3/h. Figure 6(b) shows the comparison of the variation of the predicated and measured carbon content. At the beginning of the calculation, there is a short platform period for [C] content in molten steel. This is because the decarburization reaction mainly occurs in the RH vacuum chamber, and the monitor is set in the ladle, so it takes some time to complete [C] transmission. The decarburization reaction is faster in the early stage; at about 5 minutes, the [C] content in molten steel decreased from 300 to 70 ppm; then, with the decrease of [C] and [O] content in molten steel, the decarburization rate also decreases. The simulation results are in good agreement with the industrial test results, which indicates that the current decarburization reaction model can simulate the decarburization process of molten steel in the actual RH vacuum refining process.
Carbon and Oxygen Transport
Figures 7 and 8 show the contour of [C] and [O] concentration variation with time on the central section of RH. All results shown in this chapter do not consider the oxygen blowing. It can be seen that the decrease of [C] concentration first occurs in the vacuum chamber, and then the carbon content in the whole ladle gradually decreases with the progress of decarburization reaction and the transport of carbon with the molten steel flow and concentration difference. At 600 seconds, the [C] content in the ladle has reached a low level. Finally, with the decarburization reaction, the [C] and [O] in molten steel have reached a plateau, and the concentration difference between vacuum chamber and ladle has become negligible.
Figure 9 shows the decarburization reaction rate in the central section and gas–liquid interface (αl = 0.9). The decarburization reaction mainly occurs on the surface of the argon bubbles in the up-leg snorkel, inside the molten pool and the surface of the molten steel in the vacuum chamber. In addition, when the reaction rate of decarburization in molten steel decreases, the reaction volume also decreases, which is due to the decrease of [C] content in molten steel caused by decarburization.
To increase the decarburization rate, more attention should be paied to the vacuum chamber, such as increasing the contact area between the molten steel and the vacuum environment in the vacuum chamber, and strengthen the stirring to enhance the transport of carbon and oxygen. Compared with the molten steel surface and argon bubble surface in the vacuum chamber, the whole reaction area in the molten pool is larger, especially in the corresponding area of the up-leg snorkel in the vacuum chamber. Since the decarburization reaction only takes place in the vacuum chamber and up-leg snorkel, that increasing the RH circulation flow rate makes the molten steel flow to drive the transport of [C] and [O] is also important to improve the decarburization efficiency.
According to the carbon content difference in each cell between the current time step and the previous time step, the apparent decarburization rate is obtained by the formula [46].
The apparent decarburization reaction rate of molten steel in RH at different times is shown in Figure 10. The results of 10 to 60 seconds in the figure correspond to the legend on the upper, and the results of 120 to 600 seconds correspond to the legend on the lower. In the first 60 seconds, the molten steel with low [C] content in the vacuum chamber flows into the ladle from the vacuum chamber, and then gradually flows and diffuses to the ladle wall, which is consistent with the flow cycle of molten steel, and finally leads to the apparent decarburization effect of reducing [C] content in the ladle. At the beginning of the reaction, the apparent decarburization rate of the molten steel flowing into the ladle from the down-leg snorkel is higher, because the decarburization rate of the vacuum chamber is faster at the beginning stage, the carbon content in the molten steel decreases faster, while the molten steel with lower carbon content flows into the ladle through the down-leg snorkel following the fluid flow, leading the apparent decarburization rate of molten steel at the outlet of the down-leg snorkel is higher. After 120 seconds, the apparent decarburization rate decreased by about an order of magnitude, and the distribution of [C] content in the whole RH vacuum chamber and ladle tended to be uniform, which is introduced by the flow and diffusion. The real decarburization reaction mainly occurs in the up-leg snorkel and the vacuum chamber, and the volume of molten steel in the vacuum chamber accounts for less than 10 pct of the total volume of molten steel, therefore the molten steel transfer between the vacuum chamber and the ladle will directly affect the decarburization reaction rate of the whole molten steel. The real chemical reaction mainly occurs in the vacuum chamber, and with the fluid flow and mass transfer process, the carbon content in the whole molten steel decreases uniformly.
Effect of Oxygen Blowing on Decarburization Rate
To study the influence of the top oxygen supply system on the RH decarburization reaction, four conditions of oxygen supply were compared: no oxygen blowing, oxygen blowing at the beginning of decarburization, oxygen supply after 3 and 5 minutes decarburization.
Figure 11 shows the variation of the carbon content in the molten steel at the monitor (point-(0, 800 mm, 2800 mm)) under four oxygen supply conditions. It can be seen that no matter when oxygen blowing, oxygen blowing operation can promote the decarburization process of RH refining.
To compare the contribution of three decarburization sites to total decarburization, the percentage of decarburization accumulated in the total amount of the three reaction sites was compared, as shown in Figure 12. The internal reaction of molten steel contributes the most to the decarburization, accounting for about 55.9 pct of the total. The argon bubble surface and the free surface of the vacuum chamber contribute 32.5 and 11.6 pct, respectively.
The results show that the proportion of decarburization reaction on the surface of the molten steel in the vacuum chamber increases when the oxygen supply operation was employed. Accordingly, the proportion of the reaction inside the molten steel in the vacuum chamber and the decarbonization reaction on the surface of the argon bubble in the up-leg is slightly reduced. With the postponing of the oxygen supply beginning time, the proportion of decarburization on the surface of vacuum chamber steel decreases gradually. When the oxygen supply at the beginning of the decarburization, the contribution of the free surface of the molten steel in the vacuum chamber increased from 11.6 to 28.3 pct. The promoting effect of oxygen blowing on the decarburization process is more obvious when the carbon content is relatively high. When the carbon content is very low, the transportation of the carbon in the molten steel is the restricted step in the decarburization reaction. Thus, the earlier starting blow oxygen, the more intense the decarburization reaction on the surface. When the carbon content in the molten steel is high, the oxygen blowing operation can significantly promote the decarburization reaction. When the carbon content in the molten steel is low, improving the circulation flow rate of the molten steel is more conducive to the further reduction of the carbon content in the molten steel in the ladle of the RH.
Conclusions
In the current study, the decarburization reaction in the RH vacuum refining process was simulated. The oxygen dissolution from the surface of molten steel in the vacuum chamber was considered. The variation of [C] concentration in the RH reactor and the decarburization reaction rate in each region were obtained.
-
(1)
In the case of without oxygen blowing, the decarburization reaction inside of the molten steel in the vacuum chamber has the largest contribution to the overall decarburization amount, accounting for 55.9 pct, while the proportion of the argon bubble surface and the molten steel free surface in the vacuum chamber are 32.5 and 11.6 pct, respectively.
-
(2)
The decarburization reaction mainly occurs in the RH vacuum chamber, and fluid flow and mass transfer drive the reduction of carbon content in RH ladle, so as to reduce the carbon content in molten steel in the Whole RH reactor.
-
(3)
No matter when oxygen blowing, this operation can promote the decarburization process of RH refining. The increase of oxygen supply operation leads to the increase of the proportion of decarburization reaction on the surface of molten steel in the vacuum chamber, which can increase the percentage of decarburization reaction on the surface of molten steel in the vacuum chamber from 11.6 to 28.3 pct.
-
(4)
When the carbon content in the molten steel is high, the oxygen blowing operation can significantly promote the decarburization reaction. When the carbon content in the molten steel is low, improving the circulation flow rate of the molten steel is more conducive to the further reduction of the carbon content in the molten steel in the ladle of the RH.
Abbreviations
- \(a_{{[{\text{C}}]}}\) :
-
Carbon activity in molten steel (wt pct)
- \(a_{{[{\text{O}}]}}\) :
-
Oxygen activity in molten steel (wt pct)
- A :
-
Reaction area (m2)
- A S :
-
Area of decarburization reaction on the surface of vacuum chamber (m2)
- Ak C :
-
Volumetric mass transfer coefficient of carbon (m3/s)
- Ak O :
-
Volumetric mass transfer coefficient of oxygen (m3/s)
- d b :
-
Bubble diameter (m)
- D C :
-
Diffusion coefficient of carbon (m2/s)
- D O :
-
Diffusion coefficient of oxygen (m2/s)
- \(e_{i}^{j}\) :
-
Activity interaction coefficient
- f [C] :
-
Activity coefficient of [C]
- f [O] :
-
Activity coefficient of [O]
- G :
-
Flow rate of lifting gas (NL/min)
- \(\Delta G^{\Theta }\) :
-
Gibbs free energy (J/mol)
- k C :
-
Mass transfer coefficient of carbon (m/s)
- k O :
-
Mass transfer coefficient of oxygen (m/s)
- K :
-
Equilibrium constant of carbon oxygen reaction
- K C :
-
Decarburization reaction rate constant (s−1)
- K O :
-
Deoxidation reaction rate constant (s−1)
- M :
-
Quality of molten steel with decarburization reaction (kg)
- M C :
-
Molar mass of carbon (g/mol)
- M O :
-
Molar mass of oxygen (g/mol)
- \(n_{{{\text{Ar}}}}^{{\text{b}}}\) :
-
Molar amount of argon in the bubble
- \(n_{{{\text{CO}}}}^{{\text{b}}}\) :
-
Molar amount of CO generated on the bubble surface (mol)
- N :
-
Number of gas injection nozzles
- P :
-
Pressure (Pa)
- P Θ :
-
Standard atmospheric pressure (Pa)
- P CO :
-
Partial pressure of CO (Pa)
- \(P_{{{\text{CO}}_{{2}} }}\) :
-
Partial pressure of CO2 (Pa)
- \(P_{{{\text{O}}_{{2}} }}\) :
-
Partial pressure of O2 (Pa)
- P local :
-
Local pressure (Pa)
- P critical :
-
Estimated pressure to overcome for CO bubble nucleation (Pa)
- r CO :
-
Bubble radius of generated CO (m)
- R :
-
Constant = 8.314 [J/(mol K)]
- S [C] :
-
Source term of carbon mass concentration change caused by decarburization reaction
- S [O] :
-
Source term of oxygen mass concentration change caused by decarbonization reaction and oxygen blowing process
- Sc t :
-
Turbulent Schmidt number
- t e :
-
Contact time between bubble and molten steel (s)
- T :
-
Temperature (K)
- u l :
-
Velocity of liquid phase (m/s)
- u b :
-
Bubble velocity (m/s)
- u down :
-
Longitudinal velocity of the down-leg snorkel (m/s)
- u R :
-
Relative velocity (m/s)
- V interior :
-
Volume of molten steel with decarburization reaction inside the molten steel (m3)
- V surface :
-
Volume of molten steel with decarburization reaction on the surface of molten steel (m3)
- V bubble :
-
Volume of molten steel at the corresponding position of decarburization reaction of bubbles (m3)
- ε :
-
Turbulent dissipation rate (m2/s3)
- μ t :
-
Turbulent viscosity [kg/(m s)]
- ρ :
-
Density (kg/m3)
- σ gl :
-
Surface tension between gas and liquid (N/m)
References
Y. Shan, J. Miettinen, S. Lei, and S. Louhenkilpi: Steel Res. Int., 2015, vol. 86, pp. 466–77.
J. Wei, S. Zhu, and N. Yu: Ironmaking Steelmaking., 2000, vol. 27, pp. 129–37.
K. Kleimt: Metall. Secondaire., 1995, vol. 92, pp. 493–502.
B. Kleimt, J. Cappel, J. Hoffmann, D. Sheng, and S. Landa: Revue De Métall., 2003, vol. 100, pp. 583–93.
H. Ling and L. Zhang: Metall. Mater. Trans. B., 2018, vol. 49B, pp. 2709–21.
L. Gao, Z. Shi, Y. Yang, D. Li, G. Zhang, A. Mclean, and K. Chattopadhyay: Metall. Mater. Trans. B., 2018, vol. 49B, pp. 1985–94.
T. Kitamura, K. Miyamoto, R. Tsujino, S. Mizoguchi, and K. Kato: ISIJ Int., 1996, vol. 36, pp. 395–401.
B. Liu, G. Zhu, H. Li, B. Li, Y. Cui, and A. Cui: Int. J. Min. Metall. Mater., 2010, vol. 17, pp. 22–27.
P. Li, Q. Wu, W. Hu, and J. Ye: J. Iron Steel Res. Int., 2015, vol. 22, pp. 63–67.
H. Yoshihiko, I. Hiroshi, and S. Yoshiyasu: Tetsu-to-hagané., 1998, vol. 84, pp. 21–26.
C. Lin, Y. Liu, C. Chang, J. Feng, C. Tseng, W. Chou, and C. Tai: China Steel Technical Repot., 2016, vol. 29, pp. 8–14.
S. Pirker and K. Forstner: Steel Res. Int., 2008, vol. 79, pp. 591–99.
D. Zhan, Y. Zhang, Z. Jiang, and H. Zhang: J. Iron Steel Res. Int., 2018, vol. 25, pp. 409–16.
T. Kuwabara, K. Umezawa, K. Mori, and H. Watanabe: Trans. Iron Steel Inst. Jpn., 1988, vol. 28, pp. 305–14.
S. Inoue, Y. Furuno, T. Usui, and S. Miyahara: ISIJ Int., 1992, vol. 32, pp. 120–25.
L. Zhang, X. Jing, J. Li, T. Xu, and K. Cai: Int. J. Miner. Metall. Mater., 1997, vol. 4, pp. 19–23.
Y. Park and K. Yi: ISIJ Int., 2003, vol. 43, pp. 1403–09.
H. Ling and L. Zhang: Metall. Mater. Trans. B., 2018, vol. 49B, pp. 2963–68.
J. Zhang, L. Liu, X. Zhao, S. Lei, and Q. Dong: ISIJ Int., 2014, vol. 54, pp. 1560–69.
H. Ling, F. Li, L. Zhang, and A. Conejo: Metall. Mater. Trans. B., 2016, vol. 47B, pp. 1950–61.
Y. Luo, C. Liu, Y. Ren and L. Zhang: Steel Res. Int., 2018, pp. 201800048.
H. Ling and L. Zhang: Metall. Mater. Trans. B., 2019, vol. 50, pp. 2017–28.
C. Liu, H. Duan, and L. Zhang: Metals., 2019, vol. 9, p. 442.
J. Zhang: in Metallurgical Physicochemistry. Metallurgical Industry Press, 2004, pp. 307–35.
H. Suito and R. Inoue: Trans. Iron Steel Inst. Jpn., 1996, vol. 36, pp. 528–36.
J. Wang, L. Zhang, T. Wen, Y. Ren, and W. Yang: Metall. Mater. Trans. B., 2021, https://doi.org/10.1007/s11663-021-02120-x.
Y. Huang, G. Cheng, Q. Wang, S. Li, and W. Dai: Ironmaking Steelmaking., 2019, vol. 47, pp. 655–64.
X. Wang: in Iron and Steel Metallurgy: Steelmaking. Higher Education Press, 2007.
J. Wei, N. Yu, Y. Fan, S. Yang, J. Ma, and D. Zhu: J of Shanghai University (English Edition)., 2002, vol. 6, pp. 167–75.
D. Geng, J. Zheng, K. Wang, P. Wang, R. Liang, H. Liu, H. Lei, and J.C. He: Metall. Mater. Trans. B., 2015, vol. 46B, pp. 1484–93.
Acknowledgments
The authors are grateful for support from the National Natural Science Foundation of China (Grant No. U1860206, No. 51725402), the Fundamental Research Funds for the Central Universities (Grant No. FRF-BD-20-04A), the High Steel Center (HSC) at Yanshan University, Beijing International Center of Advanced and Intelligent Manufacturing of High Quality Steel Materials (ICSM), the High Quality Steel Consortium (HQSC) of University of Science and Technology Beijing, China.
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Manuscript submitted September 14, 2021; accepted March 26, 2022.
Rights and permissions
About this article
Cite this article
Peng, K., Liu, C., Zhang, L. et al. Numerical Simulation of Decarburization Reaction with Oxygen Blowing During RH Refining Process. Metall Mater Trans B 53, 2004–2017 (2022). https://doi.org/10.1007/s11663-022-02519-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-022-02519-0