Abstract
Ni-based superalloy, which has excellent high-temperature strength and corrosion resistance, is mainly used in aviation materials, high-performance internal combustion engines, and turbines for thermal and nuclear power generation. For this reason, refining the impurities in Ni-based superalloys is a very important technical task. Nevertheless, the original technology for the melting and refining of Ni-based superalloys is still insufficient. Therefore, in this study, the effect of the CaO-Al2O3-MgO-TiO2 slag on the removal efficiency of an impurity element sulfur in Incoloy® 825 superalloy, one of the representative Ni-based superalloys, was investigated. The desulfurization behavior according to the change of TiO2 content and CaO/Al2O3 (=C/A, basicity) ratio as experimental variables was observed at 1773 K (1500 °C). Although the TiO2 content in the slag increases to 15 mass pct, the mass transfer coefficient of sulfur in molten alloy showed a constant value. Alternatively, under the condition of C/A > 1.0 of slag, the mass transfer coefficient of sulfur showed a constant value, whereas under the condition of C/A < 1.0, the mass transfer coefficient of sulfur greatly decreased as CaO decreased. Hence, in the desulfurization of Incoloy® 825 superalloy using the CaO-Al2O3-MgO-TiO2 slag, the TiO2 content in the slag does not have a considerable effect on the desulfurization rate and desulfurization mechanism (metal phase mass transfer controlled regime), but the basicity of the slag has a significant effect on desulfurization mechanism. When the slag basicity decreases below the critical level, i.e., C/A < 1.0, which is corresponding to sulfur distribution ratio, Ls < 200, it was confirmed that the desulfurization mechanism shifts from the metal phase mass transfer-controlled regime to the slag phase mass transfer-controlled regime due to the variation in the physicochemical properties of the slag such as viscosity and sulfide capacity. In addition, the different desulfurization rates between steel and Ni alloy melts were discussed by employing the diffusivity of sulfur in both systems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nickel-based superalloys usually have high strength and corrosion resistance at severe environment with high temperatures. Due to this property, they have been used as aviation material, high-performance internal combustion engine, thermal power, and nuclear power turbine. However, because the impurities, such as sulfur, phosphorous, oxygen, and nitrogen can react with the host metal or other alloying elements to form precipitates that have detrimental effects on physical properties of the final product, the removal of these impurity elements is highly important for use of Ni-based alloys in the above applications.[1,2] In particular, sulfur adversely affects tensile ductility, stress rupture life, and crack propagation resistance in Ni-based superalloys.[2,3]
Since the 1970s, in order to improve the quality and performance of Ni-based superalloys, many studies have been conducted to remove sulfur from alloy melts.[4,5,6] Utada et al.[7] reported the ‘direct and complete recycling,’ which is the method that cast a single crystal alloy using a CaO crucible and adjusting the composition of the melt. Degawa and Ototani [8] also investigated desulfurization behavior of Ni-based superalloy, Inconel® 738 using calcia (CaO) refractory. They concluded that calcia refractory is very effective not only in desulfurization but also in deoxidation as well as nitrogen removal process. Kishimoto et al.[9] reported the desulfurization model in molten Ni-based superalloy by using solid CaO rod and calculated the effective sulfur diffusivity in molten Ni-based superalloy. Also, they found that CaO and Al2O3 generate solid calcium aluminates, which capture CaS to remove S from the melt at 1673 K (1400 °C), while CaO and Al2O3 form the solid–liquid coexisting calcium aluminates, which capture CaS and penetrate the particle boundaries of the CaO rod to remove S from the melt at 1773 K and 1873 K (1500 °C and 1600 °C).[10] Moreover, they suggested that the rate-determining process of the desulfurization reaction is sulfur diffusion in the generated calcium aluminates layer at any temperature.
Refining of Ni-based superalloys is typically performed by vacuum induction melting (VIM) and electroslag remelting (ESR) in industry. Zhang et al.[11] investigated the desulfurization behavior of Ni-based superalloy, in which Al was added as desulfurizer using VIM method. Niu and Hu [12] reported the nitrogen removal mechanism in Ni-based superalloy during VIM and calculated the activity coefficient of N (\(f_{{\text{N}}} \; = \;0.714\)). On the other hand, the ESR process, which is a method of refining metals employing a molten slag that is heated by electrical resistance, has been adopted as a refining process to produce ultra-clean Ni-based alloys.[13] Eissa and El-Mohammadi [14] confirmed that high degree of desulfurization of steels can be attained by using the 70CaF2-15CaO-15Al2O3 (mass pct) ESR slag system. Yang and Park [15] studied the ESR slag design to control Al and Ti contents in the Ni-based alloy using ESR process. Duan et al.[16] reported that increasing TiO2 content in the ESR slag decreased the desulfurization efficiency of Inconel® 718 alloy.
Alternatively, Kellner et al.[17] investigated the evolution of non-metallic inclusions from FeTi70 ferroalloy and Incoloy® 825 superalloy melt through the high-temperature experiments, which have similarity with ladle refining process in steel industry. Nevertheless, there are few studies on ladle refining concept of Ni-based superalloys. Comprehensively, although the special melting and refining processes such as VIM and ESR produce high-quality alloys, the process cost is generally higher than commercial ladle refining technology.
Therefore, in the present study, along with the pursuit of reducing the production cost due to the VIM and ESR processes, the applicability of the desulfurization of Ni-based superalloy using CaO-based ladle slag was investigated. The desulfurization behavior of Ni-based superalloy using the CaO-Al2O3-MgO-TiO2 slags with different TiO2 contents and different CaO/Al2O3 ratios on the desulfurization kinetics of Incoloy® 825 superalloy was conducted at 1773 K (1500 °C).
Experimental Procedure
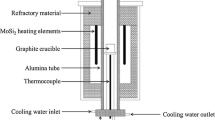
The experiments were performed using a high-frequency induction furnace (Figure 1). The quartz reaction chamber was initially evacuated prior to carrying out the experiments and the chamber was thereafter filled with a purified Ar-3 pct H2 gas mixture. Impurities in the Ar-3 pct H2 gas mixture were removed by passing the gas through Drierite® (W.A Hammond Drierite Co. Ltd., Xenia, OH), silica gel, soda lime, and Mg turnings at 773 K (500 °C).
The Incoloy® 825 superalloy (500 g) was put into a fused MgO crucible (51 mm inner diameter, 60 mm outer diameter, 100 mm height) with a graphite heater, which was surrounded by insulation. The composition of Incoloy® 825 superalloy used in the present experiments is listed in Table I. The experimental temperature was 1773[± 2] K (1500[± 2] °C), which was regulated using a proportional–integral–differential controller and a B-type (Pt-30Rh/Pt-6Rh, mass pct) thermocouple.
After the temperature stabilization was confirmed, slag (50 g), which was prepared using a vertical resistance furnace with a mixture of reagent grade CaO, calcined from CaCO3 at 1273 K (1000 °C), Al2O3, MgO, and TiO2, was injected through the quartz tube. The compositions of the pre-fused slags are listed in Table II and are plotted in the CaO-Al2O3-TiO2-5 pct MgO phase diagram (Figure 2) with iso-activity CaO contours, which were constructed from FactSageTM7.3 calculations.
In situ suction sampling and quenching were employed to obtain alloy and slag samples during the metal–slag reactions at 1773 K (1500 °C) at various time intervals (0, 5, 10, 20, 30, 60 minutes). The sulfur content in the slag and metal samples was measured using a combustion analyzer (CS 800, ELTRA). By using an inductively coupled plasma-atomic emission spectrometry (ICP-AES, ACROS, SPECTRO), the alloy composition was obtained. The experimental results according to the slag compositions are listed in Table II. The saturation level of MgO in the present slag system was calculated to (MgO)sat=6–8 mass pct using FactSageTM7.3 software with FToxid database and was experimentally confirmed that the slag composition was not seriously different from the initial one within the analytical error range, i.e., ± 2 pct. Hence, the slag composition is expressed as initial one in the present study. The equilibrium sulfur content and sulfur distribution ratio in each condition were obtained in the separate experiments, which are described elsewhere.[18]
In the present slag system, the ionic speciation of titanium oxide, which is dependent on the oxygen chemical potential, was confirmed by employing an X-ray photoelectron spectroscopy (XPS, K-alpha (mono); Thermo UK) analysis with quenched slag samples. From XPS spectra for Ti 2p shown in Figure 3, it was confirmed that the oxidation state of Ti was \({\text{Ti}}^{4 + }\) in the present CaO-Al2O3-TiO2-MgO slag system from the observation of \({\text{Ti}}^{4 + }\) peaks (458.5 eV). The representative binding energy is shown in Table III.[15,19] Hence, through the 458.5 eV peak in Figure 3, it can be reasonably assumed that titanium is mainly stabilized as TiO2 in the present slag system under the present experimental conditions.
Results and Discussion
Thermodynamics of Sulfur Removal from Incoloy® 825 Superalloy: Sulfide Capacity and Sulfur Distribution Ratio
The equilibrium S distribution ratio (= \(L_{s}\) = (mass pct S) / [mass pct S]) between metal and slag is used to evaluate the desulfurization efficiency regarding thermodynamics in iron- and steelmaking processes. In order to investigate the influence of sulfide capacity (= \(C_{{S^{2 - } }}\)) and desulfurization conditions on \(L_{s}\), the sulfide capacity of the slag was calculated from Eq. [3] deduced from desulfurization reactions, Eqs. [1] and [2].
here \(a_{{\text{O}}}\) is the activity of oxygen in molten alloy, determined by the Al-Al2O3 equilibrium reaction according to Eq. [4]:[5]
and \(f_{S}\) is the activity coefficient of S in molten Incoloy® 825 superalloy, which was assumed to be unity due to scarcity of thermodynamic data, e.g., interaction parameters for all solute elements, in Ni-based melts. The calculated activity of oxygen in molten alloy and that of Al2O3 in the slag are listed in Table IV.
The sulfide capacity \(C_{{s^{2 - } }}\) was also calculated using the FactSageTM7.3 software,[20,21] a commercial thermochemical computing package which has been widely used to obtain the equilibria of multicomponent, and multiphase metallurgical systems.[22,23,24,25,26,27,28,29,30,31] The effect of the different TiO2 content and C/A ratio on \(C_{{s^{2 - } }}\) can be compared and the calculation results are plotted as iso-capacity contours in Figure 4. It can be easily found that the iso-capacity contours exhibit nearly the same tendency with the iso-\(a_{CaO}\) contour in the present slag system: i) Sulfide capacity strongly dependent on the CaO content, ii) Sulfide capacity contours are parallel to the CaO saturation liquidus line, iii) Effect of TiO2 content on sulfide capacity is less significant than that of CaO, and iv) Sulfide capacity slightly decreases with increasing TiO2 content at a given C/A ratio (but not significant).
Moreover, for the calculation of sulfide capacity with various TiO2 contents and C/A ratios, a relationship between sulfide capacity and theoretical optical basicity reported by several authors was employed. Duffy and Ingram[32,33,34] defined the optical basicity as the ability of an oxygen anion to provide negative charge to the oxide melts. The optical basicity of slags can be calculated from Eq. [6]:
where \(x_{i}\) is mole fraction of oxide component \(i\), \(n_{i}\) is the number of oxygen in component of each oxide \(i\) , and \(\Lambda_{i}\) is optical basicity of pure oxide component \(i\), i.e., \(\Lambda_{{{\text{CaO}}}} = 1.0\), \(\Lambda_{{{\text{TiO}}_{2} }} = 0.61\), \(\Lambda_{{{\text{Al}}_{2} {\text{O}}_{3} }} = 0.60\), and \(\Lambda_{{{\text{MgO}}}} = 0.78\). Sosinsky and Sommerville[35] obtained a correlation between sulfide capacity and optical basicity such as Eq. [7] at temperatures from 1673 K (1400 °C) to 1973 K (1700 °C):
The sulfide capacity calculated from FactSageTM7.3 software, experimental results, and calculated from Sosinsky and Sommerville equation, Eq. [7] are compared in Figure 5. From Figures 4 and 5, there is no significant change in sulfide capacity due to the changes in TiO2 content. Although titanium oxide has multivalence nature, of which ionic speciation could be affected by the oxygen potential as well as aluminate structure, it is obvious that TiO2 has minor impact on sulfide capacity of the present slag system. However, the structural role of titanium oxide in the calcium aluminate-based melts should be investigated by employing the spectroscopic methodology, for example, Raman spectroscopy,[25] which is beyond the scope of the present study. On the other hand, the sulfide capacity continuously increases as the CaO/Al2O3 ratio increases. The scatters in three kinds of estimations are not significant and thus the sulfide capacity calculations from measured results in the present study are within the acceptable window.
The measured sulfur distribution ratios between Incoloy® 825 superalloy and slag, \(L_{s}\) at 1773 K (1500 °C) are represented in Figure 6. As similar to the sulfide capacity, there is no significant deviation in \(L_{s}\) by varying TiO2 content to 15 mass pct, whereas \(L_{s}\) significantly increases from approx. \(L_{s}\)= 40 to 570 as C/A ratio increases from C/A = 0.6 to 1.3. By employing the thermodynamic behavior of sulfur between Incoloy® 825 superalloy and the CaO-Al2O3-5 pct MgO-TiO2 slag, the kinetics of sulfur removal from Incoloy® 825 superalloy melt will be quantitatively discussed in the following section.
Desulfurization Kinetics of Incoloy® 825 Superalloy via Various Slag Compositions
Sulfur concentration in Incoloy® 825 superalloy melt through fully liquid slag with reaction time is shown in Figure 7 for various slag compositions such as TiO2 content [Figure 7(a)] and CaO/Al2O3 ratio [Figure 7(b)]. In our previous studies in molten steel, it was confirmed that the sulfur concentration approaches the equilibrium value within 10 to 15 minutes.[36,37] However, in the present study, the sulfur concentration rapidly decreases within 20 to 30 minutes. As shown in Figure 7(a), there is no considerable difference in desulfurization rate according to the change in the TiO2 content, whereas the desulfurization rate is significantly affected by the C/A ratio as shown in Figure 7(b).
It has been proposed that the overall desulfurization rate in secondary refining processes is mainly controlled by mass transfer of sulfur in metal phase due to the relatively high slag basicity, i.e., high sulfide capacity as well as rapid interfacial chemical reactions at high temperatures.[38,39,40,41,42,43] Also, the desulfurization rate equation is generally expressed as 1st-order reaction to sulfur concentration in the metal phase as given in Eqs. [8] and [9]:[38,39,43]
where \(C_{o}\), \(C_{t}\), \(k\) , and \(t\) are, respectively, the initial sulfur concentration in liquid metal at time \(t\)=0, the S concentration at time \(t = t\), the apparent 1st-order rate constant (s-1), and the reaction time (s). The apparent rate constant, \(k\) calculated from Eq. [9] is shown as a function of TiO2 content and C/A ratio of the slag in Figure 8. The apparent rate constant does not vary by TiO2 content, while it is strongly affected by C/A ratio of the slag. Specifically, the apparent rate constant sharply decreases by decreasing the C/A ratio at C/A < 1.0 regime.
For sulfur mass transfer, flux through the metal and slag phases can be expressed as follows:[43]
Under the steady state conditions, i.e., \(J_{m} = J_{s}\), the overall desulfurization flux (\(J\)) and overall mass transfer coefficient (kO) values can be deduced in Eqs. [12] and [13] below:
where \(J\), \(C\), \(V\), \(A\), \(k_{O}\), \(t\), and \(\rho\), respectively, represent the molar flux of sulfur (mol m-2 s-1), the molar concentration of sulfur (mol m-3), the volume (m3), the reaction area (m2), the overall mass transfer coefficient (m s-1), time (sec), and density (kg m-3). Superscripts \(b\) and \(i\) indicate the bulk and interface, while subscripts \(m\) and \(s\) indicate the metal and slag phase, respectively. If the desulfurization rate can be determined through mixed mass transfer in the metal and slag phases both, the slag–metal reaction can be regarded as a coupled reaction without prioritization to either metal phase control or slag phase control as the rate-controlling step (RCS). Therefore, the overall mass transfer coefficient can be represented in Eq. [13]. The denominator in Eq. [13] can be considered as the total resistance to mass transfer, where \(\frac{{\rho_{m} }}{{\rho_{s} k_{s} L_{S} }}\) is the resistance in the slag phase and \(\frac{1}{{k_{m} }}\) is the resistance in the metal phase. Thus, the overall mass transfer coefficient can be deduced by either \(k_{m}\) or \(k_{s} L_{s} \frac{{\rho_{s} }}{{\rho_{m} }}\) depending on the variation in mass transfer coefficients between the metal and slag phase, and/or the value of \(L_{s}\).
By integrating Eqs. [10] and [11], the following rate formulas in Eqs. [14] and [15] could be deduced:
where \(W\) and \(\left[ S \right]\) represent the weight (kg) and S concentration in the metal phase (mass pct), respectively. The subscripts \(o\) and \(e\) represent initial (\(t = 0\)) and equilibrium (\(t = t_{eq}\)) conditions, respectively. The metal phase mass transfer coefficient, \(k_{m}\), according to the slag compositions are shown in Figure 9. Despite the increase of TiO2 in the slag, \(k_{m}\) showed almost constant value, whereas \(k_{m}\) significantly decreases as C/A ratio decreases at C/A < 1.0. This indicates that TiO2 does not affect the desulfurization mechanism in metal phase mass transfer-controlled regime, while the transition of RCS occurs from metal phase mass transfer to slag phase mass transfer or mixed-controlled regime as decreasing C/A ratio at C/A < 1.0.
The relationship between the overall mass transfer coefficient, \(k_{O}\) (obtained from Eq. [13]) and the sulfide capacity as well as viscosity of the slag are shown in Figure 10. In Figure 10(a), the overall mass transfer coefficient \(k_{O}\) linearly increases with increasing sulfide capacity of the slag on a logarithmic scale. In addition, the viscosity of the slag was calculated using FactSageTM7.3 software. As shown in Figure 10(b), the viscosity (η) does not affect the \(k_{O}\) value at η < 2 dPa s (poise), while \(k_{O}\) significantly decreases with the increase in viscosity of the slag at η > 2 dPa s.
Meanwhile, the slag phase mass transfer coefficient \(k_{s}\) can be expressed as the following Eq. [16][41,42,43]:
where \(D\) and \(\delta\) , respectively, represent the diffusion coefficient (m2 s-1) and diffusion boundary layer (m). Here, the diffusion coefficient of sulfur \(D\) can be expressed as follows according to the Stokes–Einstein equation [17].[44]
where \(k_{B}\) is Boltzmann’s constant (1.38×10-23 J K-1), \(T\) is temperature (K), \(\eta\) is the viscosity of slag (dPa s), and \(r\) is the radius of the spherical particle (m). From Eq. [17], diffusion coefficient of sulfur \(D\) is inversely proportional to slag viscosity at a given temperature, which means that the slag phase mass transfer coefficient \(k_{s}\) are also inversely affected by slag viscosity from Eqs. [16] and [17].
Jeong et al.[37] investigated the characteristics of slag by considering the thermochemical and thermophysical properties of slag, i.e., ‘\(\log C_{{S^{2 - } }} - \log \eta\)’ to evaluate the mass transfer coefficient of sulfur in the desulfurization process of molten steel. In the present study, one more thermodynamic parameter, \(a_{O}\) (the activity of oxygen in the Incoloy® 825 superalloy) is employed, resulting in the ‘\(\log C_{s} - \log {\eta } - \log a_{O}\),’ because the distribution ratio of sulfur, \(L_{s}\), which is one of the dominant factors affecting the overall mass transfer coefficient from Eq. [13], is dependent on the activity of oxygen in the molten alloy as given in Eq. [3]. The overall mass transfer coefficient \(logk_{O}\) is plotted as a function of physicochemical parameter ‘\(\log C_{s} - \log {\eta } - \log a_{O}\)’ in Figure 11, wherein a good linear relationship can be obtained with the following equation from a least square regression method.
From this linear relationship, the thermochemical properties such as sulfide capacity of slag as well as the activity of oxygen in molten alloy in conjunction with the thermophysical property such as slag viscosity are combinatorial factors controlling sulfur removal mechanism.
The relationship between the equilibrium sulfur distribution ratio and the overall mass transfer coefficient is presented in Figure 12. Solid circles represent the current experimental results (i.e., the overall mass transfer coefficient, \(k_{O}\), calculated from Eq. [13]), and black solid line represents the metal phase mass transfer coefficient measured in the present study \(\left( {\log k_{m} = - 4.3} \right)\). Open triangles and a grey dashed line were obtained from Kang et al.’s study,[36] in which the desulfurization experiments were performed on steel melt under the similar conditions to that of present study, i.e., the same dimension of crucible assembly with same slag/metal ratio (=0.1) using an identical induction furnace, and similar CaO-Al2O3-MgO-based slag system. A different condition is temperature: Kang et al.[36] performed desulfurization experiment at 1823 K (1550 °C). Thus, their experimental data were extrapolated to 1773 K (1500 °C) from an Arrhenius equation (i.e., activation energy) they reported.
Relationship between the mass transfer coefficients of sulfur and equilibrium sulfur distribution ratio in the molten Ni-based alloy. The metal phase mass transfer coefficient of sulfur in molten steel is also compared[36]
Kang et al.[36] concluded that the overall desulfurization rate was determined by the mass transfer of sulfur in the metal phase when the equilibrium S distribution ratio is higher than about \(L_{{\text{S}}} = 200\) and deduced the metal phase mass transfer coefficient to be \(k_{{\text{m}}} = 2.0 \times 10^{ - 4} {\text{ m}}/{\text{s }}\left( {\log k_{{\text{m}}} = - 3.7} \right)\), which is four times higher than the metal phase mass transfer coefficient in Ni-based alloy melt. From Figure 12, the overall mass transfer coefficient (\(\log k_{{{O}}} )\) drastically decrease by decreasing the equilibrium S distribution ratio. The overall mass transfer coefficient, kO, approaches gradually to the metal phase mass transfer coefficient, km, under condition for the higher equilibrium S distribution ratio, i.e., \(L_{{\text{s}}} > 200\). However, the overall mass transfer coefficient rapidly converges to the \(k_{{\text{s}}} \cdot L_{{\text{S}}}\) under the condition for the lower S distribution ratio, specifically \(L_{{\text{S}}} < 200\).
Difference of Desulfurization Rate in Iron- and Nickel-Based Melts
As mentioned in the previous section, the present study for Ni-based alloy melts and Kang et al.'s work for molten steel showed a large difference in desulfurization rate, despite almost similar experimental conditions except for the difference in base metal system. From Eq. [17], the reason for the difference in the metal phase mass transfer coefficient, \(k_{m}\) is possibly caused by the difference in the diffusivity of sulfur in iron- or nickel-based alloy melts. Because the same apparatus and the similar experimental conditions were adopted in both studies, the diffusion boundary layer thickness (\(\delta\)) can be assumed to be similar in both studies. This can be indirectly corroborated by the similar convective flow of the melt due to an induction heating with the same frequency in all experiments.
Frazier et al.[45] reported the diffusion coefficient of sulfur in nickel from 1300 K to 1750 K (1027 °C to 1477 °C). From an extrapolation of Frazier et al.’s results to 1773 K to 1823 K (1500 °C to 1550 °C), the diffusion coefficient of sulfur in nickel was deduced as \(D_{{\text{S}}}^{{{\text{Ni}}}} \; = \;5.08\; \times \;10^{ - 11} \;{\text{m}}^{2} \;{\text{s}}^{ - 1}\) (1773 K [1500 °C]) to \(D_{{\text{S}}}^{{{\text{Ni}}}} \; = \;7.63\; \times \;10^{ - 11} \;{\text{m}}^{2} \;{\text{s}}^{ - 1}\) (1823 K [1550 °C]), respectively, which is about 1/60 to 1/90 of sulfur diffusion coefficient in iron, i.e., \(D_{{\text{S}}}^{{{\text{Fe}}}} \; = \;4.5\; \times \;10^{ - 9} \;{\text{m}}^{2} \;{\text{s}}^{ - 1}\) at 1823 K (1550 °C). Thus, it is certain that the diffusion coefficient of sulfur in nickel is much less than that in molten iron considering a similarity of density and viscosity of molten nickel and iron as given in Table V. Nevertheless, further research is needed to reveal a difference in the diffusion kinetics of sulfur between iron- and nickel-based melts.
Conclusions
In the present study, desulfurization behavior of Ni-based superalloy (Incoloy® 825 superalloy) melts with the CaO-Al2O3-MgO-TiO2 slag with different content of TiO2 and CaO/Al2O3 (=C/A) ratio at 1773 K (1500 °C) was investigated. Moreover, the rate-controlling step of the desulfurization in the present refining process and the transition of the rate-controlling step via slag conditions were discussed. The following is a summary of the major findings reported in the present study:
-
1.
Sulfide capacity of the slag was not changed dramatically with different TiO2 contents, while it was drastically decreased by decreasing C/A ratio. There was good correspondence between the calculated results by using FactSageTM7.3 software or using optical basicity model, and obtained results from measured sulfur distribution ratio in the present experiment.
-
2.
The metal phase mass transfer controlled regime was not affected by the changes in TiO2 content of the slag near the CaO saturation composition range. However, when the CaO/Al2O3 ratio is lower than unity, in which a sulfur distribution ratio is lower than 200, the transition of RCS from the metal phase mass transfer to the slag phase mass transfer or slag–metal combined transportation-controlled regime was observed.
-
3.
There was a difference in the desulfurization rate in nickel-based (superalloy) and iron-based (steel) melts under the similar refining conditions. This seems to be caused by the difference in a diffusivity of sulfur in Ni-based and Fe-based melts, but further research needs to be performed to reveal the quantitative analysis.
References
W.R. Sun, S.R. Guo, J.H. Lee, N.K. Park, Y.S. Yoo, S.J. Choe, and Z.Q. Hu: Mater. Sci. Eng. A., 1998, vol. 247, pp. 173–9.
J.X. Dong, X.S. Xie, and R.G. Thompson: Metall. Mater. Trans. A., 2000, vol. 31A, pp. 2135–44.
J.P. Beckman and D.A. Woodford: Proc. of Superalloys 1988, The Metallurgical Society, 1988, pp. 795-804.
G.A. Meyer, J.S. Warner, Y.K. Rao, and H.H. Kellogg: Metall. Trans. B., 1975, vol. 6B, pp. 229–35.
G.K. Sigworth, J.F. Elliott, G. Vaughn, and G.H. Geiger: Trans. Metall. Soc. CIM, 1977, pp. 104–10.
R.Y. Lin, D.C. Hu, and Y.A. Chang: Metall. Trans. A., 1978, vol. 9B, pp. 531–8.
S. Utada, Y. Joh, M. Osawa, T. Yokokawa, T. Kobayashi, K. Kawagishi, S. Suzuki, and H. Harada: Proc. of Superalloys 2016, TMS, 2016, pp. 591-99.
T. Degawa and T. Ototani: Tetsu-to-Hagane., 1987, vol. 73, pp. 1691–7.
Y. Kishimoto, S. Utada, T. Iguchi, Y. Mori, M. Osawa, T. Yokokawa, T. Kobayashi, K. Kawagishi, S. Suzuki, and H. Harada: Metall. Mater. Trans. B., 2020, vol. 51B, pp. 293–305.
Y. Kishimoto, T. Kono, T. Horie, T. Yokokawa, M. Osawa, K. Kawagishi, S. Suzuki, and H. Harada: Metall. Mater. Trans. B., 2021, vol. 52B, pp. 1450–62.
T. Zhang, Y. Wei, J. Chen, N. Li, and B. Han: J. Aust. Ceram. Soc., 2020, vol. 56, pp. 885–94.
J. Niu and Z. Hu: Adv. Mater. Res., 2011, vol. 284–286, pp. 2433–6.
R.C. Reed: The Superalloys-Fundamentals and Applications, Cambridge University Press, Cambridge, 2006, pp. 217–29.
M. Eissa and A. El-Mohammadi: Steel Res., 1998, vol. 69, pp. 413–7.
J.G. Yang and J.H. Park: Metall. Mater. Trans. B., 2017, vol. 48B, pp. 2147–56.
S.C. Duan, X. Shi, F. Wang, M.C. Zhang, B. Li, W.S. Yang, H.J. Guo, and J. Guo: J. Mater. Res. Technol., 2019, vol. 8, pp. 2508–16.
H.E.O. Kellner, A.V. Karasev, A. Memarpour, and P.G. Jonsson: Steel Res. Int., 2016, vol. 87, pp. 1461–8.
J.H. Cho, J. Martinsson, D. Sichen, and J.H. Park: to be published.
J.A. Rotole, K. Gaskell, A. Comte, and P.M.A. Sherwood: J. Vac. Sci. Technol. A., 2001, vol. 19, pp. 1178–81.
www.factsage.com. Accessed April 2021.
C.W. Bale, E. Belisle, S.A. Decterov, G. Eriksson, K. Hack, I.H. Jung, Y.B. Kang, J. Melancon, A.D. Pelton, C. Robelin, and S. Petersen: Calphad., 2009, vol. 33, pp. 295–311.
J.H. Park: J. Non-Cryst. Solids., 2012, vol. 358, pp. 3096–102.
J.S. Park and J.H. Park: Steel Res. Int., 2014, vol. 85, pp. 1303–9.
J.H. Park, I.H. Jung, and S.B. Lee: Met. Mater. Int., 2009, vol. 15, pp. 677–81.
Y.B. Kang and J.H. Park: Metall. Mater. Trans. B., 2011, vol. 42B, pp. 1211–7.
J.H. Heo, S.S. Park, and J.H. Park: Metall. Mater. Trans. B., 2012, vol. 43B, pp. 1098–105.
J.H. Park: Calphad., 2007, vol. 31, pp. 149–54.
S.K. Kwon, Y.M. Kong, and J.H. Park: Met. Mater. Int., 2014, vol. 20, pp. 959–66.
J.H. Park, G.H. Park, and Y.E. Lee: ISIJ Int., 2010, vol. 50, pp. 1078–83.
J.H. Shin and J.H. Park: Metall. Mater. Trans. B., 2017, vol. 48B, pp. 2820–5.
J.H. Park: J. Am. Ceram. Soc., 2006, vol. 89, pp. 608–15.
J.A. Duffy and M.D. Ingram: J. Am. Chem. Soc., 1971, vol. 93, pp. 6448–54.
J.A. Duffy: J. Non-Cryst. Solids., 1989, vol. 109, pp. 35–9.
J.A. Duffy: J. Chem. Educ., 1996, vol. 73, pp. 1138–42.
D.J. Sosinsky and I.D. Sommerville: Metall. Trans. B., 1986, vol. 17B, pp. 331–7.
J.G. Kang, J.H. Shin, Y.S. Chung, and J.H. Park: Metall. Mater. Trans. B., 2017, vol. 48B, pp. 2123–35.
T.S. Jeong and J.H. Park: Metall. Mater. Trans. B., 2020, vol. 51B, pp. 2309–19.
F. Oeters: Metallurgie der Stahlherstellung, Verlag Stahleisen GmbH, Dusseldorf, and Springer, Berlin, 1994.
J.X. Deng and F. Oeters: Proc. 7th Japan-Germany Seminar on Fundamentals of Iron and Steelmaking, Verlag Stahleisen GmbH, Dusseldorf, 1987, p. 33.
Y.H. Kim: Ph.D. Dissertation, University of Strathclyde, Strathclyde, Glasgow, UK, 1978.
B. Deo and P. Grieveson: Steel Res., 1986, vol. 57, pp. 514–9.
S. Ramachandran, T.B. King, and N.J. Grant: Trans. Metall. Soc. AIME., 1956, vol. 206, pp. 1549–53.
B. Deo and R. Boom: Fundamentals of Steelmaking Metallurgy, Prentice Hall, New York, 1993.
A. Einstein: Ann. Phys., 1905, vol. 17, pp. 549–60.
W.E. Frazier, T.T. Mickle, and B.A. Pregger: Research Report, Naval Air Development Center, Department of The Navy, 1993.
T. Saito, Y. Kawai, K. Maruya, and M. Maki: Sci. Rep. Inst. Tohoku Univ. Ser. A., 1959, vol. 11, pp. 401–10.
M. Watanabe, M. Adachi, and H. Fukuyama: J. Mater. Sci., 2016, vol. 51, pp. 3303–10.
Y. Sato, K. Sugisawa, D. Aooki, and T. Yamamura: Meas. Sci. Technol., 2005, vol. 16, pp. 363–71.
Acknowledgments
This work was supported by the Competency Development Program for Industry Specialists from the Korea Institute for Advancement of Technology (KIAT, Grant Number P0002019) and the Korea Evaluation Institute of Industrial Technology (KEIT, Grant Number 20009956), funded by the Ministry of Trade, Industry and Energy (MOTIE), Korea. Also, this study was partly supported by the LG Yonam Foundation, Korea.
Funding
Open access funding provided by Royal Institute of Technology.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Manuscript submitted May 31, 2021; accepted September 19, 2021.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cho, J.H., Martinsson, J., Sichen, D. et al. Desulfurization Behavior of Incoloy® 825 Superalloy by CaO-Al2O3-MgO-TiO2 Slag. Metall Mater Trans B 52, 3660–3670 (2021). https://doi.org/10.1007/s11663-021-02338-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-021-02338-9