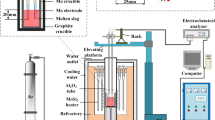
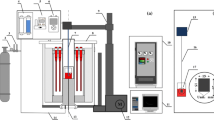
Abstract
The effect of Al2O3 and Al2O3/SiO2 ratio on the sulfide capacity of the molten aluminosilicate CaO-SiO2-Al2O3-MgO-TiO2 slag system with high Al2O3 content was measured at 1773 K (1500 °C) using a metal-slag equilibration method. The sulfide capacity between silicate-based and aluminate-based slag was also compared based on the thermodynamic analysis and structural characteristics of melts. At a fixed CaO/SiO2 ratio of 1.20, the sulfide capacity decreases with increasing Al2O3 content primarily due to the decrease of free oxygen (FO) and the activity of O2–. Increasing the Al2O3/SiO2 ratio from 0.47 to 0.79 causes a significant increase in the sulfide capacity of the slags, and a slight increase is found when the Al2O3/SiO2 ratio is more than 0.79. The effect of the substitution of silica by alumina on the sulfide capacity of the slags was not only due to an increase in the activity of basic oxides (\( a_{{{\text{O}}^{2 - } }} \)) but also to a decrease in the stability of sulfide (\( \gamma_{{{\text{S}}^{2 - } }} \)). Moreover, \( a_{{{\text{O}}^{2 - } }} \) and \( \gamma_{{{\text{S}}^{2 - } }} \) increase in a similar degree, and the weaker binding electronegativity of Al3+ with oxygen atoms results in a slight increase in the final sulfide capacity in the aluminate-based slag system with Al2O3 ↔ SiO2 substitution. Five different sulfide capacity models were employed to predict the sulfide capacity, and the iso-sulfide capacity distribution diagram based on the Young’s model was obtained in the high Al2O3 corner of the diagram.









Similar content being viewed by others
References
J.H. Park and G.H. Park: ISIJ Int., 2012, vol. 52, pp. 764–69.
G.H. Park, Y.B. Kang, and J.H. Park: ISIJ Int., 2011, vol. 51, pp. 1375–82.
M.K. Cho, J. Cheng, J.H. Park, and D.J. Min: ISIJ Int., 2010, vol. 50, pp. 215–21.
T. Tanaka, Y. Ogiso, M. Ueda, and J. Lee: Tetsu-to-Hagane, 2009, vol. 95, pp. A275–A281.
J. Zhang, X. Lv, Z. Yan, Y. Qin, and C. Bai: Ironmaking & Steelmaking, 2016, vol. 5, pp. 378–84.
A. Shankar, M. Gornerup, A.K. Lahiri, and S. Seetharaman: Metall. Mater. Trans. B, 2006, vol. 37B, pp. 941–47.
Y. Taniguchi, L.J. Wang, N. Sano, and S. Seetharaman: Metall. Mater. Trans B, 2012, vol. 43B, pp. 477–84.
S.J. Jeong, T.S. Kim, and J.H. Park: Metall. Mater. Trans. B, 2017, vol. 48B, pp. 545–53.
Y. Gao, Q. Liu, and L. Bian: Metall. Mater. Trans. B, 2012, vol. 43B, pp. 229–32.
P. Yan, X. Guo, S. Huang, J. Dyck, M. Guo, and B. Blanpain: ISIJ Int., 2013, vol. 53, pp. 459–67.
L. Wang, M. Hayashi, K.C. Chou, and S. Seetharaman: Metall. Mater. Trans. B, 2012, vol. 43B, pp. 1338–43.
R. Moretti and G. Ottonello: Metall. Mater. Trans. B, 2003, vol. 34B, pp. 399–410.
D. Liang, Z. Yan, X. Lv, J. Zhang, and C. Bai: Metall. Mater. Trans. B, 2017, vol. 48B, pp. 573–81.
Z. Yan, X. Lv, D. Liang, J. Zhang, and C. Bai: Metall. Mater. Trans. B, 2016, vol. 47B, pp. 1–8.
M.M. Nzotta, M. Andreasson, P. Jonsson, and S. Seetharaman: Scand. J. Metall., 2000, vol. 29, pp. 177–84.
M.M. Nzotta, D. Sichen, and S. Seetharaman: Metall. Mater. Trans. B, 1999, vol. 30B, pp. 909–20.
M.M. Nzotta, D. Sichen, and S. Seetharaman: ISIJ Int., 1998, vol. 38, pp. 1170–79.
M.M. Nzotta, R. Nilsson, D. Sichen, and S. Seetharaman: Ironmaking & Steelmaking, 1997, vol. 24, pp. 300–05.
E. Drakaliysky, N.S. Srinivasan, and L.I. Staffansson: Scand. J. Metall., 1991, vol. 20 (4), pp. 251–55.
D.J. Sosinsky and I.D. Sommerville: Metall. Mater. Trans. B, 1986, vol. 17B, pp. 331–37.
R. Young, J. Duffy, G. Hassall, and Z. Xu: Ironmaking and Steelmaking, 1992, vol. 19, pp. 265–68.
G. Zhang, K. Chou, and U. Pal: ISIJ Int., 2013, vol. 53, pp. 761–67.
www.factsage.com (accessed Sept. 2011)|.
G.G. Hatch and J. Chipman: J. Met., 1949, vol. 1, pp. 274–80.
E.T. Turkdogan: Metall. Mater. Trans. B, 1978, vol. 9B, pp. 163–79.
A. Shankar: Ironmaking and Steelmaking, 2006, vol. 33, pp. 413–18.
E.T. Turkdogan: Physical Chemistry of High Temperature Technology, Academic Press, New York, NY, 1980, pp. 1–24.
K. Kim, W.W. Huh, and D.J. Min: Metall. Mater. Trans. B, 2013, vol. 45B, pp. 889–96.
X. Tang and C. Xu: ISIJ Int., 1995, vol. 35, pp. 367–71.
C. Shi, X. Yang, J. Jiao, C. Li, and H. Guo: ISIJ Int., 2010, vol. 50, pp. 1362–72.
D.H. Woo and H.G. Lee: J. Am. Ceram. Soc., 2010, vol. 93, pp. 2098–2106.
C.J. Fincham and F.D. Richardson: Proc. R. Soc. A: Math., Phys., Eng. Sci., 1954, vol. 223, pp. 40–62.
J.A. Duffy and M.D. Ingram: J. Am. Chem. Soc., 1971, vol. 93, pp. 6448–54.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (Grant No. 51234010), Program for New Century Excellent Talents in University, and Program for the Youth Top-notch Talents of Chongqing (Grant No. 20151001).
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted March 21, 2017.
Rights and permissions
About this article
Cite this article
Yan, Z., Lv, X., Pang, Z. et al. Transition of Blast Furnace Slag from Silicate Based to Aluminate Based: Sulfide Capacity. Metall Mater Trans B 48, 2607–2614 (2017). https://doi.org/10.1007/s11663-017-1031-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-017-1031-8