Abstract
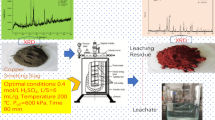
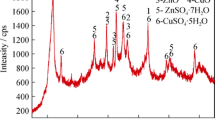
Although ammonia leaching of copper from slags has been reported generally as a part of copper slag utilization methods, but no detailed studies have been reported in the literature. In this research, we tried to investigate the effect of different parameters on ammonia leaching of copper from copper smelting slag by identifying different copper-bearing phases and following them during leaching time. Mineralogical characterization of the smelting slag (1.7 pct Cu) was done using X-ray fluorescence, X-ray diffraction, optical microscopy, diagnostic leaching tests, and scanning electron microscopy. The characterization studies indicated that main copper-bearing species are soluble copper oxides and chalcocite along with minor amount of covellite, bornite, blister copper particles, and chalcopyrite. It was also found that only approximately 0.2 pct Cu was present in the insoluble bulk silicate phases. These results suggest that approximately 88 pct of the total copper of slag could be extracted by ammonia sulfide leaching. Leaching tests were carried out and the effects of various parameters, namely pH, ammonia concentration, temperature, presence of oxygen, stirring speed, and pulp density were examined on copper leaching. The temperature and stirring speed had the most pronounced effect on the copper leaching, whereas ammonia affected the leaching yield at low concentrations of ammonia. It was found that 78 pct of Cu could be extracted within 4 hours and under optimum conditions: T = 343 K (70 °C), 2M ammonia, pH 10.5, stirring speed = 900 rpm, pulp density = 10 pct (w s/v). The kinetic data were analyzed with the shrinking core models, and it was found that the leaching process is controlled by both the interfacial transfer and diffusion across the product layer and the activation energy is calculated to be 49.4 kJ mol−1.













Similar content being viewed by others
References
N.M. Piatak, M.B. Parsons, and R.R. Seal: Appl. Geochem., 2015, vol. 57, pp. 236-266.
B. Gorai, R.K. Jana, and Premchand: Resourc. Conserv. Recyc., 2003, vol. 39, pp. 299-313.
Y. Li, I. Perederiy, and V.G. Papangelakis: J. Hazard. Mater., 2008, vol. 152, pp. 607-05.
A. Rozendaal and R. Horn: Miner. Eng., 2013, vol. 52, pp. 184-90.
K.-H. Park, D. Mohapatra, B. Ramachandra Reddy, and C.-W. Nam: Hydrometallurgy, 2007, vol. 86, pp. 164–71.
G.A. Parkison: SME Preprint Number 95-90 (SME, Denver, CO 1995).
W.W. Fisher: Miner. Eng., 1994, vol. 7, pp. 99-103.
Q. Yin, D.J. Vaughan, K.E.R. England, and G.H. Kelsall: J. Colloid Interf. Sci., 1994, vol. 166, pp. 133–42.
A.N. Buckley, I.C. Hamilton, and R. Woods: J. Appl. Electrochem., 1984, vol. 14, pp. 63-74.
Z. Liu, Z. Yin, S. Xiong, Y. Chen, and Q. Chen: Hydrometallurgy, 2014, vol 144-145, pp. 86–90.
Z.-X. Liu, Z.-L. Yin, H.P. Hu, and Q.-Y. Chen: J. Cent. South Univ. Technol., 2012, vol. 19, pp. 77-84.
Y. Konishi, M. Katoh, and S. Asai: MTB, 1991, vol. 22, pp. 295–303.
D. Tromans: Miner. Eng., 2000, vol. 13, pp. 497–515.
L.W. Beckstead and J.D. Miller: MTB, 1977, vol. 8, pp. 31–38.
15. M.I. Muravyov, N.V. Fomchenko, A.V. Usoltsev, E.A. Vasilyev, and T.F. Kondrat’eva: Hydrometallurgy, 2012, vols. 119–120, pp. 40–46.
M.L. Free: Hydrometallurgy, John Wiley & Sons, Inc., New York, NY, 2013, pp. 84–136.
C.F. Dickinson and G.R. Heal: Thermochim. Acta, 1999, vols. 340–341, pp. 89–103.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Manuscript submitted November 3, 2014.
Rights and permissions
About this article
Cite this article
Bidari, E., Aghazadeh, V. Investigation of Copper Ammonia Leaching from Smelter Slags: Characterization, Leaching and Kinetics. Metall Mater Trans B 46, 2305–2314 (2015). https://doi.org/10.1007/s11663-015-0394-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-015-0394-y