Abstract
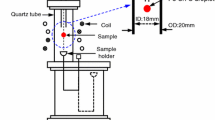
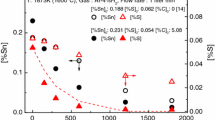
Evaporation of Sn from molten steel was experimentally investigated for Fe-Sn-S alloy with low initial S (0.0007 < [pct S]0 < 0.05) or with high initial S (0.55 < [pct S]0 < 0.894) at 1873 K (1600 °C) using an electromagnetic levitation melting technique, in order to clarify the role of S on the evaporation mechanism of Sn. It was found that increasing initial S concentration, [pct S]0, decreased the second-order evaporation rate constant of Sn (k SnS), but there was a residual rate for the evaporation even at high [pct S]0. The obtained residual rate constant, \( k_{\text{SnS}}^{\text{r}} \), was 1.4 × 10−9 m4 mol−1 s−1 at 1873 K (1600 °C). Evaporation of Sn under virtually no S condition ([pct S]0 = 0.0007) was also measured and corresponding first-order rate constant was determined to be 3.49 × 10−7 m s−1 at 1873 K (1600 °C). A comprehensive model for the Sn evaporation from molten Fe-Sn-S alloy was developed in the present study, under the condition where mass transfer in gas and liquid phases were fast and interfacial chemical reaction controlled the evaporation of Sn. The model equation is able to represent the evaporation of Sn in the forms of Sn(g) and SnS(g) simultaneously, from very low S melt (when there is no S) to very high S melt investigated in the present study up to ~0.9 mass pct. Gradual transition of major evaporation species from SnS(g) to Sn(g) was well accounted for by the developed model.











Similar content being viewed by others
Abbreviations
- ρ :
-
Density of liquid alloy, kg m−3
- A :
-
Area of reaction surface, m2
- i :
-
Species i dissolved in liquid alloy
- i i :
-
Species i at the interface
- [pct i]:
-
Mass percent of species i in liquid alloy at time t
- [pct i]0 :
-
Mass percent of species i in liquid alloy at initial state (t = 0)
- k Sn :
-
Apparent rate constant of a first-order reaction, m s−1
- \( k_{\text{Sn}}^{\text{R}} \) :
-
Chemical reaction rate constant of the Reaction Sn = Sn(g) when surface of liquid alloy is fully open, m s−1
- k SnS :
-
Apparent rate constant of a second-order reaction, m s−1
- \( k_{\text{SnS}}^{\text{R}} \) :
-
Chemical reaction rate constant of the Reaction Sn i + S i = SnS(g)i when surface of liquid alloy is fully open, m4 mol−1 s−1
- \( k_{\text{SnS}}^{\text{r}} \) :
-
Residual rate constant of the Reaction Sn i + S i = SnS(g)i, m4 mol−1 s−1
- K S :
-
Adsorption coefficient of S
- M i :
-
Molecular weight of element i, kg mol−1
- n i :
-
Number of moles of species i, mol
- t :
-
Reaction time, s
- V :
-
Volume of liquid alloy, m3
References
S.-H. Jung, Y.-B. Kang, J.-D. Seo, J.-K. Park, and J. Choi: Metall. Mater. Trans. B, 2014, DOI:10.1007/s11663-014-0163-3.
V.D. Sehgal: J. Iron Steel Inst., 1970, vol. 208, pp. 382-386.
R. Morales D. and N. Sano: Ironmaking and Steelmaking, 1982, vol. 9, pp. 64-76.
L. Savov and D. Janke: ISIJ Int., 2000, vol. 40, pp. 654-663.
X. Liu and J.H.E. Jeffes: Ironmaking and Steelmaking, 1988, vol. 15, pp. 21-26.
X. Liu and J.H.E. Jeffes: Ironmaking and Steelmaking, 1988, vol. 15, pp. 27-32.
I. Langmuir: J. Amer. Chem. Soc., 1918, vol. 40, pp. 1361-1403.
D.R. Sain and G.R. Belton: Metall. Trans. B, 1976, vol. 7B, pp. 235-244.
D.R. Sain and G.R. Belton: Metall. Trans. B, 1978, vol. 9B, pp. 403-407.
S.-H. Jung, Y.-B. Kang, J.-D. Seo, J.-K. Park, and J. Choi: unpublished research, 2014.
K. Sekino, T. Nagasaka, and R.J. Fruehan: ISIJ Int., 2000, vol. 40, pp. 315-321.
H.-G. Lee and Y.K. Rao: Metall. Trans. B, 1982, vol. 13B, pp. 411-421.
L. Savov and D. Janke: ISIJ Int., 2000, vol. 40, pp. 95-104.
Acknowledgments
This research was financially supported by POSCO Ltd. through Steel Innovation Program.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted February 24, 2014.
Rights and permissions
About this article
Cite this article
Jung, SH., Kang, YB., Seo, JD. et al. Evaporation Mechanism of Sn and SnS from Liquid Fe: Part II: Residual Site and Evaporation Kinetics via Sn(g) and SnS(g). Metall Mater Trans B 46, 259–266 (2015). https://doi.org/10.1007/s11663-014-0177-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-014-0177-x