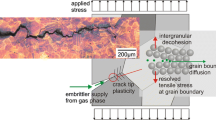
Abstract
The crack propagation properties are an important and often limiting factor for the commercial application of superalloys in aerospace applications where the oxidation ahead of the crack tip plays an important role. Previous research on the Ni-base superalloy A718Plus revealed that the orientation and the volume fraction of the high temperature grain boundary phases δ and η have a major influence on the dwell fatigue crack propagation rate at 650 °C in ambient air but not in vacuum. In this work, the effect of internal oxidation at the η/δ–matrix interface and its effect on dwell fatigue crack propagation and mechanical properties are examined by advanced microscopic methods and micro-cantilever testing. During crack propagation, the crack tip is exposed to air where a Nb-rich oxide layer forms and embrittles the η/δ–matrix interface causing the cracks to deflect and propagate along the oxidized interfaces. Micro-cantilever tests on the oxidized interface show that these oxide layers also significantly reduce the local strength and fracture toughness of the material. This proves that interfacial oxide layers are the underlying reason for the reduction of the dwell fatigue crack growth resistance at 650 °C, particularly in microstructures whose η/δ–matrix interfaces are oriented parallel to the crack growth direction.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In modern society, complex materials for special technical applications are essential. The in-depth understanding of these materials is a key for innovation and new technology. One such material is the polycrystalline Ni-base superalloy A718Plus, which is based on the commercially widely used Inconel 718 and which can be used for rotating components in aircraft engines and gas power plants.[1] Besides the main hardening L12 Ni3Al γ′ phase, the hexagonal D024 Ni3Ti or Ni6AlNb η and the orthorhombic D0a Ni3Nb δ high temperature phases were proven to precipitate in A718Plus, mostly in plate-like morphology, which serve as high temperature grain boundary pinning phases.[1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19] Depending on the heat-treatment procedure, either the η or δ phase prevails.[4] They appear often in a lamellar arrangement with a specific crystallographic orientation relationship and precipitate beside and inside each other.[9,20,21] Therefore, they are hereafter referred to as η/δ precipitates. In contrast to Inconel 718, typically no γ′′ precipitates are present, while γ′ precipitates are stabilized in A718Plus. Accordingly, the alloy has a higher thermal stability, since coarsening and dissolution of γ′ and γ′′ as well as the transformation of γ′′ to δ deteriorate the microstructure of Inconel 718 at high temperatures, limiting its service temperature to 650 °C.[22,23,24] In an earlier work, it was shown that a correlation between the crack propagation rate and the orientation of the high temperature grain boundary η/δ precipitates exists.[2] If the precipitates are orientated perpendicular to the crack propagation direction, the dwell crack propagation resistance increases in comparison to a random orientation of the η/δ plates. If the η/δ plates are oriented parallel to the crack propagation direction, the dwell crack propagation resistance is reduced (Figure 1). Further measurements of the crack propagation rate revealed that the sensitivity with respect to the orientation of the η/δ plates exists only when the tests are carried out in air and is absent for tests in vacuum.[2] In comparison to the commercially used Ni-base superalloy Waspaloy, which is free of any grain boundary phases, A718Plus shows a much higher crack propagation rate during dwell fatigue at 704 °C in air, while in an inert vacuum atmosphere, the two superalloys are nearly identical.[19] This leads to the assumption that the oxidation ahead of the crack tip, especially at the η/δ-γ matrix interface, plays a critical role for the crack propagation rate of A718Plus. The influence of grain boundary precipitates and oxidation on the crack propagation of Ni-base superalloys has been analyzed in the literature before.[19,25,26,27] Earlier studies on A718Plus showed the influence of other microstructural factors like grain size, η/δ volume fraction, γ′ size, and volume fraction on the fatigue crack growth resistance and minimum creep rate.[28] From these results, it can be concluded that although these parameters have an influence on the dwell fatigue crack propagation of A718Plus, the effect of the orientation of the η/δ precipitates in air is by far bigger, as shown in Figure 1(c)). At 650 °C in air, the crack propagation rate in A718Plus is by a factor of about 103 faster than in vacuum.[2] Study on the parent alloy IN718 tested between 560 and 725 °C already revealed that grain boundary precipitates decrease the resistance against crack propagation of IN718 due to oxidation at interfaces, where Nb enriches and facilitates growth of a brittle Nb-oxide.[27]
Microstructure and crack propagation behavior of A718Plus. (a) Microstructure with random orientation of the η/δ precipitates. (b) Microstructure with aligned η/δ precipitates. The arrows indicate the crack propagation in good (green) and bad (blue) direction relative to the precipitate orientation. Reprinted with permission from Ref. [2]. (c) Influence of alignment on the fatigue resistance at 650 °C in air of A718Plus (red) compared with the effect of other microstructural properties (light red area) as well as literature values for the commercially used Ni-base superalloys IN718 (black), U720Li (light gray), and Waspaloy (dark gray) at the same temperature. Adapted from Ref. [28] (Color figure online)
Accordingly, the present study conducts a detailed analysis on the interaction of cracks with η/δ precipitates, the influence of oxidation ahead of the crack tip as well as the effect of interfacial oxides on the mechanical properties. In combination with earlier studies[2,16] and the findings of this work, a comprehensive overview of the effect of different microstructural features on dwell crack propagation is given.
2 Experimental Methods
The material used in this study was provided by Rolls-Royce Deutschland Ltd & Co Kg (RRD, Deutschland), which was processed by Allegheny Technologies Incorporated (ATI) into billets and has been hot formed at Otto Fuchs KG (Germany) into a near turbine disc form. The composition is given in Table I. The material was processed as described in References [2,16] to precipitate the η/δ phase in an oriented plate-like morphology.
Additionally, Rolls-Royce Deutschland Ltd. & Co. KG provided an already tested A718Plus compact tension sample (CT) which is used for fatigue crack measurements in a specified area of the sample according to ASTM and ISO standards. The CT specimen was tested at 650 °C with a 1-600-1-1 cycle as outlined in Figure 2. The stress was applied perpendicular to the η/δ plate orientation. That way, the orientation of the η/δ precipitates is parallel to the anticipated crack propagation direction.
The crack and the oxide-affected region surrounding the crack were visualized using a Zeiss Crossbeam 540 scanning electron microscope (SEM) in backscattered electrons (BSE) mode with an acceleration voltage of 20 kV at a working distance of 8.5 mm. The same microscope was used for scanning transmission electron microscopy (STEM) and energy disperse X-ray spectroscopy (EDS). The working distance for STEM was set to 2 mm, and the distance between the electron column and the detector was set to 12 mm with an acceleration voltage of 30 kV and a current of 350 pA. The EDS measurements were done on the STEM foils with an Oxford instrument detector using 5 kV acceleration voltage. The STEM foil was cut using a FEI Helios NanoLab 600i focused ion beam (FIB) system.
An oxidized and a non-oxidized condition of A718Plus was investigated by performing micro-cantilever testing in order to test the influence of oxidation on the cracking behavior. Therefore, one A718Plus sample was additionally heat treated at 800 °C/500 h in air to ensure a complete and deep oxidation of the surface and along the η/δ interfaces underneath. Afterward, the FEI Helios NanoLab 600i was used to prepare micro-cantilevers with an aspect ratio of 2:2:10 µm (height:width:length) from the oxidation influenced zone close to the surface of this sample as well as from another non-oxidized sample for comparison. Prior to the milling of the micro-cantilevers with a Ga+-ion beam at 30 kV, the samples were ground and polished. An η/δ plate with a vertical orientation was embedded in the complete cross section of the cantilevers. The rough milling was done with a high current of 2.5 nA and the fine preparation of the exact geometry with a current of 80 pA. A notch with a depth of about 650 nm was cut at the interface between an η/δ plate and the γ matrix with a current of 24 pA. The fracture behavior was determined at ambient temperature by in situ bending with the force measurement system Kleindiek FMS-EM inside a Zeiss Crossbeam 1540. The FMS-EM system consists of a micromanipulator MM3A-EM and a force sensitive cantilever with a piezoresistive coating. A video is recorded to track the displacement of the cantilever by digital image correlation (DIC) with the software Veddac 6.0 from Chemnitzer Werkstoffmechanik GmbH.
The stress intensity factor KIC of materials with a small plastic zone can be calculated from the maximum force during loading and the dimensions of the cantilever[29] according to the following equation.
where F is the applied load, and L is the distance between loading position of the indenter tip and the notch. The geometry function f depends on the ratio of the initial crack length a, the width W, and thickness B of the cantilever.[30]
As the fracture experiments showed plastic material behavior, the J-integral introduced by Rice[31] was used. The J-Integral was calculated with a simplified version of the iterative method given in ASTM 1820.[32]
where Jel and Jpl represent the elastic and plastic part of the J-integral for each iteration step i, Young’s modulus E, and the Poisson’s ratio ν of the material describes Jel. For the plain strain state, the dimensionless number η is two.[32] The plastic work Apl was determined numerically from the load displacement curves for each point, subtracting the elastic work by using the initial loading slope k. It must be considered that plain strain conditions were presumed for all cantilevers.
To transform the J-integral into the fracture toughness KIQ,J, the following equation was used:
A Young’s modulus E of 213 GPa and the Poisson’s ratio of 0.31 for A718Plus were used for the calculations. The value of the J-integral was determined at the maximum applied force.
3 Results
3.1 Crack Propagation and Interaction with η/δ Precipitates
The microstructure of the CT sample with crack propagation along the orientated η/δ precipitates is shown in Figure 3. The main and secondary cracks are clearly visible. In Figure 3(a1)), the crack propagates perpendicular to the applied stress axis, following mainly the η/δ–γ matrix interface. In the η/δ-free matrix area, the crack propagates along the grain boundaries, as seen in the EBSD measurement (Figure 3(a2)), until it hits the next η/δ precipitate and gets deflected again. A more detailed image of the grown oxide and propagation of the crack along η/δ precipitates is shown in Figure 3(b), which depicts the crack tip of this sample. Oxidation seems to occur at the η/δ − η/δ interface as well as the η/δ–γ matrix interface ahead of the crack, as indicated by the blue arrows.
3.2 Characterization of the Oxides
To investigate the oxide phases at the crack as well as grain and phase boundaries in detail, STEM and STEM EDS investigations were conducted. For this, a TEM foil was cut which included a part of the primary crack. Figure 4(a) shows an oxidized crack and δ/η precipitates as well as the oxidized η/δ− γ matrix interface in a dark field STEM image. Oxide formation is visible at the η/δ precipitates and at different interfaces. The oxide layer observed at the η/δ precipitates was deep and partly consumed the precipitate during oxidation. Compared to the γ matrix there seems to be a higher oxidation rate of the η/δ precipitates and of the interfaces as highlighted by yellow arrows. Furthermore, oxidation takes place along grain boundaries, which is highlighted by the green arrow.
To analyze the chemical composition of the oxides, EDS measurements were conducted. Figure 4(b) through (i) shows Ni, Co, Nb, Cr, Fe, Al, Mo, and O EDS maps, indicating different oxide phases depending on the microstructure surrounding the oxidized crack. The oxidized area is visualized in Figure 4(i) by the increased O concentration. The mappings clearly show the enrichment of Ni, Nb, and Al in the η/δ phase.
The oxidized crack surface is enriched in Cr and O and depleted in Fe as can be seen from Figure 4. Interestingly, Mo shows also a slight accumulating at the γ matrix− η/δ interface, while Al seems to have no significant effect on the oxidation at all. It has to be noted that no oxygen or other oxidizing element can be measured at the presumably oxidized grain boundary indicated by the green arrow. This is due to the reduced resolution of the EDS detector that was used compared to the STEM detector. Additionally, the extremely small thickness of the sample necessary for STEM analysis reduces the signal strength of the EDS detector, further reducing the resolution. Due to the clear change in contrast seen in Figure 4(a)) under the consideration of Figure 4(i) which shows that an increase in O brightens the contrast in the STEM image, the assumption can be made, that the grain boundary shows oxidation, even though it is not visible in the EDS subfigures.
The crack propagates along a γ matrix− η/δ interface where a high Nb content is present. To quantify these findings, EDS linescans on three different regions of the oxidized crack were conducted (Figure 5(a)). Line 1 (Figure 5(b)) displays the concentration profile across the oxidized crack at a η/δ–matrix interface. Three different oxide layers can be determined. Directly at the η/δ precipitate–crack interface, a Nb-rich oxide forms which has grown into the η/δ-precipitate and consumes Nb from the Nb-rich η/δ phase. In the inner region of the oxidized crack, a Ni and Co enrichment is found with nearly no Nb. At the former crack–γ matrix interface, Ni and Co are reduced, while Cr is enriched which indicates a Cr-oxide layer. In comparison to that, line 2 (Figure 5(c)) shows the oxide layer in a region with no adjacent η/δ phase. At the crack–matrix interface, a slight increase of Cr and a decrease of Ni can be detected, whereas the inner region exhibits a higher Ni and a reduced Cr content, similar to line 1. Line 3 (Figure 5(d)) across a narrow secondary crack reveals only Cr-oxide.
(a) EDS linescans across the oxidized crack. The η/δ precipitates are indicated by the yellow dashed lines. (b) Crack adjacent to a η/δ precipitate. (c) Crack with no adjacent η/δ phase. (d) Secondary crack without adjacent η/δ. The red arrows indicate damage of the STEM foil while processing. These areas had to be filled with Pt and are therefore not further discussed (Color figure online)
3.3 Influence of Interfacial Oxidation on Fracture Toughness
With notched micro-cantilevers the η/δ− γ matrix interface was tested to study an influence of oxidation on crack propagation. The force displacement curves in Figure 6 show that the non-oxidized sample starts to deform plastically at a force of about 410 µN. At a displacement close to 1.5 µm, the experiment was interrupted because the measuring range of the micromanipulator was at its limit. The non-oxidized cantilevers reveal a more ductile behavior. The crack becomes blunted and a plastic zone with slip bands is visible at the crack tip. In contrast, the oxidized cantilever reveals a more brittle crack propagation behavior and only a smaller plastic zone forms at the crack tip. The crack runs along the η/δ-γ matrix interface and gets deflected into the η/δ precipitate. The reason of this deflection could be that the last part of the cantilever breaks violently through the brittle η/δ precipitates due to the reduced remaining volume. The η/δ plates within the cantilevers are indicated with yellow dashed lines in Figure 6.
(a) force-displacements curves of samples after (b) no additional heat-treatment (NHT) and (c) additional heat-treatment (HT) at 800 °C for 500 h with (b), (c) corresponding SEM image of the micro-cantilevers. The yellow marked area in the SEM images indicate the η/δ phase. The red arrows in the non-oxidized state point to glide planes (Color figure online)
The fracture toughness was determined by analyzing two cantilevers for each condition and calculating the mean from both experiments. The non-oxidized condition reveals the highest fracture toughness with more ductile behavior. It should be noted that the average fracture toughness of 8.5 MPa√m for the NHT cantilever has to be seen as a minimum value due to the limited measuring range of the micromanipulator and the small crack propagation. In contrast, the less ductile cantilever of the oxidized condition has a significantly lower fracture toughness of about 4.1 MPa√m.
4 Discussion
Depending on how wide the crack is as well as whether grain boundary η/δ precipitates are present, different oxide layers are formed, which are illustrated in Figure 7.
The negative effect of oxidation on the crack propagation properties is shown in Figure 6. The oxide phases, particularly the Nb-oxide, at η/δ–γ matrix interfaces clearly reduce the strength and strain to failure of the material locally. This reduces the crack propagation resistance of the material and explains why the cracks propagate preferentially along the oxidized η/δ–matrix interfaces. Similar mechanisms were observed by Stratulat et al.[26] in the Ni-base superalloy Inconel 600. In contrast, the non-oxidized material reveals a plastic zone, slip bands, and no crack propagation along the oxidized η/δ−γ matrix interfaces.
The oxygen diffuses faster along the grain boundaries and interfaces,[25] which explains the preferred oxidation along grain boundaries and the η/δ–γ matrix interfaces. To grow Ni- and Fe-based oxides at 650 °C in similar systems as A718Plus, oxygen partial pressures (P(O2)) of about 10 Pa are required, while for Cr-based oxides, only P(O2) of about 10-2 Pa is needed under the same conditions.[33] According to the data from Reference [34], Co-oxides need a very similar oxygen partial pressure as Ni and Fe. This explains why only Cr-oxide forms at the small secondary cracks, while Cr-, Fe-, Co-, and Ni-oxides are formed in wider regions of the crack as visible in Figure 5(b) and (c). This is consistent with the investigations of Viskari et al.[25] on the intergranular oxidation at the crack tip without adjacent η/δ precipitate. Next to a Ni enriched zone of the γ matrix surrounding the crack, an outer Cr2O3 layer was measured with an Ni(Fe)O oxide in the interior of the crack.[25] Apparently, the crack propagates along the grain boundaries, following higher oxygen diffusion paths where Cr-oxides form ahead of the crack tip, if no η/δ precipitate is adjacent. This is further affirmed by the crack which does not follow the assumed propagation path along the main stress axis but changes its propagation direction along the Ni-rich oxide (Figure 5(a)). Although oxidized matrix-matrix grain boundaries influence the crack propagation direction, too, the oxidized η/δ–γ matrix interfaces provides a preferred pathway for the crack propagation, as seen in Figure 3(a2) and Figure 4. It is assumed that first, the η/δ–γ matrix interface oxidizes ahead of the crack tip creating a brittle Nb-rich oxide layer, which locally reduces the ductility and the crack resistance as shown in Figure 6. The crack follows the path of the brittle Nb-oxides, therefore propagating along the η/δ precipitates. Apparently the formation of Nb-oxides at the Nb-rich η/δ-precipitates, which is seen in Figure 5, occurs earlier due to a lower free energy, which is in accordance to the Ellingham diagram.[35] The earlier forming oxidation of the η/δ–γ matrix interface leads to more severe microstructural weakening, which reduces the dwell crack propagation resistance along η/δ precipitates, than the oxidation does along grain boundaries. This was also theorized by Viskari et al.,[7] although no direct measurements of the η/δ–γ matrix interface were conducted in their work. Also, unlike the measurements of Viskari et al., no Al-rich oxide could be detected. This also fits well to the data of Reference [28]. In this study, the η/δ precipitate orientation shows a much greater impact on crack propagation resistance than the grain size and thus the grain boundaries. It has to be noted that studies by Tsang et al.[36] with less dwell time (100 s) showed no significant influence of the η/δ phase, which show, that a sufficient amount of dwell time is necessary for the η/δ precipitates to influence the dwell fatigue properties.
A negative influence of Nb on the dwell crack resistance was already proposed by Gao et al. and previously illustrated by the environmental sensitivity (ES), which is defined as the ratio between the crack propagation rate da/dN of the material in air and in vacuum.[37] Their investigations showed that Nb is enriched at the surface of the sample after heating above 500 °C, where it reacts with surface oxygen. This mechanism is coupled with the oxidization of NbC and γ′′ (Ni3Nb) at the grain boundary ,[37,38] where, among other Nb-oxides, mainly Nb2O5 is formed.[39] Accordingly, it was proposed that oxidation of elements such as Cr and Nb ahead of the crack tip occurs during crack growth, which seems to play a significant role of intergranular failure.[40] Here, the major sources of Nb are the grain boundary η/δ precipitates which causes the local formation of Nb-oxides and a strong ES of the investigated alloy. If the ES of A718Plus with different orientations of the δ/η precipitates is compared but otherwise same grain size and γ′ precipitates,[2] see Figure 8, the ES of the “good” orientation perpendicular to the crack propagation path is apparently vastly lower compared to that of the “bad” orientation parallel to the crack propagation path or compared to microstructures with larger grain sizes.[2,19,41] However, this is not the result of an improved resistance against oxidation ahead of the crack tip. Quite the contrary: Due to the orientation of the η/δ-plates perpendicular to the crack propagation direction and the preferred crack propagation along the oxidized η/δ–matrix interface and thus the high sensitivity to oxidation, the crack is deflected and the crack length is increased. Another factor potentially contributing to a slower crack propagation rate is the occasional presence of a smaller fraction of γ′ precipitates or a region entirely depleted of γ′ around the δ/η precipitates.[4,28] This could lead to locally reduced crack tip stresses, which could further reduce the crack growth rate. However, this phenomenon should be independent from the orientation of the δ/η precipitates. In summary, it is evident from this work that oxidation of Nb-rich δ/η precipitates ahead of the crack tip and the orientation of these precipitates significantly contribute to the dwell crack growth resistance of A71Plus.
ES of A718Plus calculated from data from the literature.[2,19,41] The number above the columns shows the grain size of the respective condition. “Good” and “bad” refer to the orientation of the δ/η phase with regard to the crack propagation path in Ref. [2]. It has to be noted that Huenert et al.[2] used a dwell time of 600 s, while Kearsey et al. and McDevitt et al.[19,41] used a dwell time of 100 s and that the ES of the “good” orientation of the δ/η precipitates is an average value from data given in Ref. [2]
5 Conclusion
A variety of methods, namely SEM, 3D FIB tomography, STEM, and micro-cantilever testing, were used to analyze the crack propagation and crack interaction with aligned plate-like η/δ precipitates of the polycrystalline Ni-base superalloy A718Plus. It is shown that the crack propagation follows preferentially the oxidized η/δ–γ matrix interface where Nb-rich oxides promote the crack propagation. The oxidation ahead of the crack tip locally reduces the strength, the material strain failure, and thus reduces the crack growth resistance. Detailed analysis of oxide phases using STEM and STEM EDS at the η/δ–γ matrix interface as well as areas with no adjacent η/δ precipitates at the crack reveal multiple oxide layers depending on the surrounding microstructure. If η/δ precipitates are adjacent to the crack, Nb from the Nb-rich η/δ precipitates forms a Nb-rich oxide layer at the η/δ–crack interface. If no η/δ precipitates are directly adjacent to the crack, a Cr-rich oxide layer forms. If the crack and therefore the availability of oxygen is sufficiently large, a third, Ni-, Fe-, and Co-rich oxide layer is formed in the interior of the crack. On secondary, thinner cracks, only Cr-oxide is formed if no η/δ precipitates are adjacent.
The effect of oxidation on the crack propagation at η/δ–matrix interface could be shown by micro-cantilever tests. A ductile fracture behavior with a plastic zone and slip bands is revealed in the not heat-treated condition without a pre-oxidized η/δ–γ matrix interface. The condition with a pre-oxidized η/δ–γ matrix interface exhibits a brittle fracture behavior, where the crack propagates along the η/δ–γ matrix interface, with no plastic zone. The measured fracture toughness explains the observed fracture behavior. This proves that oxidation and in particularly the formation of Nb-oxide at the η/δ–γ matrix interface drastically reduce the dwell crack propagation resistance of 718Plus.
References
R.L. Kennedy: Superalloys, 2005, vol. 718, pp. 1–4.
D. Huenert, M. Proebstle, A. Casanova, R. Schluetter, R. Schluetter, R. Krakow, R. Krakow, M. Buescher, P. Randelzhofer, A. Evans, K. Loehnert, T. Witulski, S. Neumeier, and C. Rae: in Superalloys 2016, M. Hardy, E. Huron, U. Glatzel, B. Griffin, B. Lewis, C. Rae, V. Seetharaman, and S. Tin, eds., Wiley, Hoboken, NJ, 2016, pp. 781–92.
A. Kirchmayer, H. Lyu, M. Pröbstle, F. Houllé, A. Förner, D. Huenert, M. Göken, P.J. Felfer, E. Bitzek, and S. Neumeier: Adv. Eng. Mater., 2020, vol. 2020, p. 2000149.
D. Hausmann, A. Förner, M. Pröbstle, D. Hünert, P. Felfer, M. Göken, and S. Neumeier: Adv. Eng. Mater., 2021, vol. 2021, p. 2100558.
A. Casanova, N. Martín-Piris, M. Hardy, and C. Rae: MATEC Web Conf., 2014, vol. 14, p. 09003.
P. Lin, V. Provenzano, G. Palumbo, T. Gabb, and J. Telesman: 7th Int. Symp. Superalloy 718 Derivative, 2010, pp. 243–53.
L. Viskari, Y. Cao, M. Norell, G. Sjöberg, and K. Stiller: Mater. Sci. Eng. A, 2011, vol. 528, pp. 2570–80.
B. Hassan and J. Corney: Mater. Sci. Technol., 2017, vol. 33, pp. 1879–89.
E.J. Pickering, H. Mathur, A. Bhowmik, O.M.D.M. Messé, J.S. Barnard, M.C. Hardy, R. Krakow, K. Loehnert, H.J. Stone, and C.M.F. Rae: Acta Mater., 2012, vol. 60, pp. 2757–69.
O. Messé, J. Barnard, E. Pickering, P. Midgley, and C. Rae: Philos. Mag., 2014, vol. 94, pp. 1132–52.
M. Springmann, W. Rothkegel, D. Huenert, and H. Schlums: 7th International Conference Low Cycle Fatigue.
R. Unocic, K. Unocic, R. Hayes, G. Daehn, and M. Mills: 7th Inernational Symposium of Superalloy 718 Deriv., 2010, p. 9.
G.A. Zickler, R. Schnitzer, R. Radis, R. Hochfellner, R. Schweins, M. Stockinger, and H. Leitner: Mater. Sci. Eng. A, 2009, vol. 523, pp. 295–303.
K. Löhnert and F. Pyczak,: 7th Int. Symp. Superalloy 718 Deriv.
R. Krakow, D.N. Johnstone, A.S. Eggeman, D. Hünert, M.C. Hardy, C.M.F. Rae, and P.A. Midgley: Acta Mater., 2017, vol. 130, pp. 271–80.
M. Pröbstle, S. Neumeier, D. Hünert, and M. Göken: in 8th International Symposium on Superalloy 718 and Derivatives, Seven Springs, 2014.
M. Kattoura, G.B. Viswanathan, S.R. Mannava, D. Qian, and V.K. Vasudevan: Metall. Mater. Trans. A, 2021, vol. 52A, pp. 3553–69.
M. Wang, Q. Deng, J. Du, Z. Tian, and J. Zhu: in 8th International Symposium on Superalloy 718 and Derivatives, Wiley-Blackwell, 2014, pp. 769–76.
R. Kearsey, J. Tsang, S. Oppenheimer, and E. McDevitt: Superalloys, 2012, vol. 2012, pp. 741–49.
R. Krakow, R.J. Bennett, D.N. Johnstone, Z. Vukmanovic, W. Solano-Alvarez, S.J. Lainé, J.F. Einsle, P.A. Midgley, C.M.F. Rae, and R. Hielscher: Proc. R. Soc. Math. Phys. Eng. Sci., 2017, vol. 473, p. 20170274.
Wei-Di Cao and Richard L. Kennedy: ATI Allvac.
T. Fedorova, J. Rösler, J. Klöwer, and B. Gehrmann: MATEC Web Conf., 2014, vol. 14, p. 01003.
M. Sundararaman, P. Mukhopadhyay, and S. Banerjee: Metall. Trans. A, 1988, vol. 19, pp. 453–65.
W.-D. Cao and R.K. Kennedy: Superalloys, Superalloys, 2004, pp. 91–99.
L. Viskari, M. Hörnqvist, K.L. Moore, Y. Cao, and K. Stiller: Acta Mater., 2013, vol. 61, pp. 3630–39.
A. Stratulat, D.E.J. Armstrong, and S.G. Roberts: Corros. Sci., 2016, vol. 104, pp. 9–16.
P. Valerio, M. Gao, and R.P. Wei: Scr. Metall. Mater., 1994, vol. 30, pp. 1269–74.
A. Kirchmayer, M. Pröbstle, D. Huenert, S. Neumeier, and M. Göken: Metall. Mater. Trans. A, 2023, vol. 54A, pp. 2219–26.
A. E399-90: ASTM Int., 1997, pp. 1–34.
K. Matoy, H. Schönherr, T. Detzel, T. Schöberl, R. Pippan, C. Motz, and G. Dehm: Thin Solid Films, 2009, vol. 518, pp. 247–56.
J.R. Rice: J. Appl. Mech., 1968, vol. 35, pp. 379–86.
A. E1820-01: ASTM International, 2001, pp. 1–46.
E. Andrieu, R. Molins, H. Ghonem, and A. Pineau: Mater. Sci. Eng. A, 1992, vol. 154, pp. 21–28.
I. Barin and G. Platzki: Thermochemical Data of Pure Substances, vol. 1, 3rd edn., John Wiley & Sons, Ltd, Weinheim, 1995.
T.B. Reed: Free Energy of Formation of Binary Compounds, MIT press, 1971.
J. Tsang, R.M. Kearsey, P. Aua, D. Seo, S. Oppenheimer, and W. Cao: Mater. High Temp., 2010, vol. 27, pp. 79–88.
M. Gao, D.J. Dwyer, and R.P. Wei: Scr. Metall. Mater., 1995, vol. 32, pp. 1169–74.
R.S. Piascik, R.P. Gangloff, and A. Saxena: Elevated Temperature Effects on Fatigue and Fracture, ASTM International, 1997.
C.F. Miller, G.W. Simmons, and R.P. Wei: Scr. Mater., 2000, vol. 42, pp. 227–32.
C.F. Miller, G.W. Simmons, and R.P. Wei: Scr. Mater., 2001, vol. 44, pp. 2405–10.
E.T. McDevitt, S.M. Oppenheimer, R.M. Kearsey, and J. Tsang: Mater. Sci. Forum, 2012, vol. 706–709, pp. 2428–33.
Acknowledgment
The work was conducted as a part of the German-funded research program RoKoTec (LuFoIV) and supported by the Bundesministerium für Wirtschaft und Technologie (BMWi) under Grant Number 20T0813.
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kirchmayer, A., Hausmann, D., Gabel, S. et al. The influence of crack and interface oxidation on dwell fatigue crack propagation behavior of the nickel-base superalloy ATI A718Plus. Metall Mater Trans A 55, 803–811 (2024). https://doi.org/10.1007/s11661-023-07286-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11661-023-07286-4