Abstract
The objective of the present work was the development of cathodic electrophoretic deposition (EPD) to obtain composite coatings of mesoporous sol–gel glass (MSGG) particles embedded in a zein matrix on Ti-13Nb-13Zr substrates. To deposit robust and repeatable coatings, a direct current EPD and pulsed direct current EPD as well as the deposition kinetics were investigated, including the deposition yield and deposition rate. The stability of the suspension was determined based on the zeta potential and conductivity. Macroscopically homogeneous coatings with a thickness of about 10 µm and various volume fractions of MSGG were subjected to further examination. Coatings were uniform, exhibiting open porosity and showing excellent adhesion to the substrates. Both zein and MSGG particles revealed an amorphous structure. The coated substrates demonstrated greater resistance to electrochemical corrosion in Ringer's electrolyte in comparison with the virgin (non-coated) substrate. The coatings showed high roughness and moderate hydrophilicity. The incubation of the coated substrates in concentrated 1.5 simulated body fluid (1.5SBF) showed the formation of carbonate hydroxyapatite. The composite coatings showed improved antibacterial properties against gram-negative E. coli and gram-positive S. aureus bacteria compared to pure zein coatings. Electrophoretic MSGG/zein composite coatings should be further investigated in terms of their osteoconductive behavior, to confirm their suitability for medical applications in orthopedics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Titanium alloys are utilized extensively in biomedical applications for various types of bone implants. Their great application versatility is related to their unique properties, such as excellent mechanical strength and low density, high resistance to electrochemical corrosion and biocompatibility, which makes them an outstanding material for biomedical applications.[1,2,3] Particular attention is currently being paid to β alloys, which have a lower elasticity modulus than α + β alloys, and higher biocompatibility.[4,5] For instance, Nb and Zr alloying elements in near-β alloys (e.g. Ti-13Nb-13Zr) are more desirable than Al or V used in α + β alloys (e.g. Ti-6Al-4V), because they are non-toxic to the organism and do not induce health ailments.[6,7] Nb is a highly biocompatible element and strong β-stabilizer.[8] As the content of this element in the alloy increases, the stability of the β phase also increases.[9,10] The presence of Nb also lowers the Young's modulus of β alloys,[11] which is especially beneficial for bone implants due to the lower stress shielding between implant devices and bone.[12] Young’s modulus of β alloys is about 25 pct lower than that of α + β alloys, e.g. it is 79 GPa for the Ti-13Nb-13Zr alloy.[13] Moreover, the presence of the β phase in the microstructure increases the ability of the alloy to harden during aging.[14] Zr is a neutral element and is often added for improving mechanical properties.[9] However, Zr may act also as a weak β-stabilizing element.[10] The phase composition of alloys from the Ti-Nb-Zr system depends on Nb and Zr content and heat treatment route. For instance, Geetha et al.[14] reported that the Ti-13Nb-13Zr alloy exhibited occurrence of the α′ phase, while Ti-20Nb-13Zr and Ti-20Nb-20Zr alloys revealed the presence of α′, α″ and β phases in β solution treated and water-quenched condition. However, after solution treatment in the α + β phase field the Ti-13Nb-13Zr and Ti-20Nb-13Zr alloys contained α′, α″ and β phases, while Ti-20Nb-20Zr alloy showed the presence of α and β phases. No presence of α” in the Ti-20Nb-20Zr alloy microstructure was due to the enrichment in Zr of the β phase. The high Zr content in this alloy significantly stabilized the β phase, lowered the martensitic start temperature (Ms) below room temperature and led to the formation of only two phases, namely α and β.
A serious problem of β alloys is the very slow process of osseointegration between implants and bone tissue.[15] However, modification of the implant surface, such as sandblasting,[16] chemical etching[17] or deposition of bioactive coatings,[18] can increase the adhesion of bone tissue to the implant surface. One important possibility of tailoring the surface properties of titanium alloys is the deposition of composite coatings involving a biodegradable polymer and bioactive ceramic particles.[19]
Zein as a biocompatible and biodegradable polymer has great potential in the biomedical field including drug delivery carriers, regenerative medicine scaffolds or for the production of coatings.[20] Useful properties that contribute to the high interest in biomedical applications of zein also include its low toxicity and antibacterial activity.[21,22,23] Zein is obtained from the natural source of corn protein.[24] It belongs to a group of alcohol-soluble proteins (prolamins).[25] Zein can be classified according to its solubility properties as: α, β, γ and δ.[26] The largest proportion of maize protein is α-zein at around 70 pct of the total, the next is γ-zein which accounts for around 20 pct of the total, while β- and δ-zein each account for about 5 pct.[27,28] To extract individual types of zein, various extraction agents are used.[29] For example, to obtain α-zein, only water and alcohol is required, while in the case of other types of zein an additional reducing agent is necessary. The α-zein structure consists of the repetitive homologous segments of α-helices.[30] That helices contain a major quantity of hydrophobic leucine, alanine and proline.[31] In addition to hydrophobic amino acids, zein also contains hydrophilic residues (glutamine). Although zein is used as a coating in biomedical applications due to its outstanding biological performance, it has low mechanical strength and limited bioactivity (bone bonding ability).[32] To enhance these properties, composite zein-based coatings incorporating bioactive inorganic ceramics, such as hydroxyapatite,[33] silica[34] and bioactive glass,[31,35] have been designed. Due to attractive properties, including bioactivity, osteoinductivity and antibacterial effects, silica-based bioactive glasses (BG) attract increasing attention for several biomedical applications.[36,37] The bioactive character of BGs in contact with physiological fluids leads to the formation of hydroxyapatite on their surfaces exhibiting strong bonding to bone.
It is known that a porous structure of bone-contacting biomaterials usually has a positive effect on the regenerative capacity of bone.[38] Thus, interest in mesoporous bioactive glasses has recently increased significantly.[39] This is largely due to presence of ordered pores with a size of 2–50 nm contributing to the large surface area of such mesoporous BG systems.[40,41] Mesoporosity can be altered for including effective interaction with bone tissue.[42] The possibility of delivering drugs (previously loaded in the mesopores) or increasing the interaction of implants with physiological fluids are other advantages of mesoporous BGs.[43]
Among the rapidly-developing coating methods for biomedical applications, electrophoretic deposition (EPD) deserves special interest, due to the possibility of co-depositing biopolymers, including zein, and bioactive glass particles.[31,44] Moreover, this method is cost-effective and easy to use.[45] EPD is an electrochemical method wherein the deposition process takes place on the surface of an electrically conductive material submerged in a colloid[46] or in suspension.[47] The particles, being the dispersed phase of the coating material, move in the liquid dispersing phase due to the effect of an applied electric field between the two electrodes.[48] Charged particles move towards one of the electrodes (substrate material) and settle on its surface.[49] In addition, EPD enables the deposition of coatings on substrates with complex profiles, and it is possible adjust the coating thickness through the applied electrical voltage and deposition time.[50,51] The coating morphology can also be tailored by controlling the process parameters.[52] The available literature indicates that EPD has not been used previously to deposit mesoporous bioactive glass/zein composite coatings on titanium alloy substrates. Previously, Ramos-Rivera et al.[35,44] investigated the EPD parameters and deposited BG/zein and CuO-doped BG/zein coatings, which showed high adhesion to AISI 316L-SS substrate and conferred osteoconductive and anti-infective properties. Meyer et al.[31] deposited homogeneous BG/zein and Cu-doped BG/zein coatings, allowing the formation of hydroxyapatite on their surface during in-vitro bioactivity tests. Rehman[53] showed that BG/zein coatings increased the resistance of a magnesium substrate to electrochemical corrosion and that hydroxyapatite crystals were formed on the coated substrate during Kokubo test. Ahmed and Ur Rehman[33] developed the EPD of hydroxyapatite/zein coatings. They showed that the coatings revealed good mechanical and biological properties, qualifying them for potential application in biomedicine.
In our previous study,[54] we focused on the development of optimal EPD parameters to obtain homogeneous zein coatings with strong adherence to titanium biomaterial substrates. The surface preparation of the substrates was shown to have a significant effect on the coating adhesion. The coatings were dense and characterized by higher roughness than the uncoated substrates, but they decreased the corrosion resistance of titanium in Ringer’s electrolyte at the temperature of 37 °C. The aim of the present study was the development of electrophoretic deposition and characterization of composite zein-based coatings incorporating mesoporous sol–gel glass (MSGG) particles. The conditions for the EPD of coatings, including the chemical composition of suspensions, deposition kinetics and process parameters were designed to deposit homogeneous coatings on Ti alloy. In addition, the effect of MSGG concentration in the suspension on coating adhesion strength, morphology and microstructure as well as surface topography was determined. The effect of the coatings on the wettability and electrochemical corrosion resistance of the alloy substrates was also examined. Antibacterial properties against gram-positive (S. aureus) and gram-negative (E. coli) bacteria were investigated. The dissolution kinetics of the MSGG particles in SBF and the apatite forming ability of the coated samples during incubation in SBF were also examined.
2 Material and Methods
A bar of Ti-13Nb-13Zr alloy with a diameter of 30 mm was delivered by Shaanxi Yunzhong Industry Development Co., Ltd., China. The chemical composition of the alloy determined by an Oxford X-MET 3000TX Portable XRF Analyzer (UK) was as follows (in wt pct): 72.4 ± 2.0 Ti, 13.2 ± 0.2 Nb, 13.9 ± 0.2 Zr and 0.5 ± 0.2 Fe. The alloy microstructure was characterized using scanning electron microscopy (SEM, Thermo Fischer Nova NanoSEM 450 microscope, the Netherlands) and phase composition was investigated by a Panalytical Empyrean DY1061 X-ray diffractometer, Malvern Panalytical, the Netherlands. The alloy microstructure was composed of fine laths that formed the acicular structure of α′ (hexagonal close packed) and α″ (orthorhombic, Cmcm space group) in β grains (size in the range from 20 to 80 µm) with body centered cubic crystallographic structure (Figure 1(a,b)). The bar was cut into 2.5 mm thick discs. The discs were ground with 1200 grit sandpaper.
MSGG with the molar composition of 70 pct SiO2–26 pct CaO–4 pct P2O5 was produced using the sol–gel method with the supramolecular chemistry approach followed by the evaporation induced self-assembly (EISA) process. Tetraethoxysilane (TEOS; Si(OC2H5)4), calcium nitrate tetra-hydrate (Ca(NO3)2*4H2O) and triethylphosphate (TEP; OP(OC2H5)3), were employed as the sources of SiO2, CaO and P2O5, respectively. The amount of these reagents was calculated based on molar glass composition. HCl solution was used as a catalyst for the hydrolysis and condensation reactions. A non-ionic amphiphilic triblock copolymer (EO20PO70EO20), also designated as Pluronic® P123, was used as a structure-directing factor. The preparation of mesoporous glass was started by completely dissolving a portion of P123 surfactant in ethanol, next 1 M HCl and the appropriate precursors were added: TEOS was added 5 min after the HCl addition, followed by the TEP portion after 30 min. The CaO precursor in the form of hydrated nitrate was added directly to the synthesis without prior dissolution in distilled water. The overall mixture was mixed for 24 h. The obtained solution was transferred in a covered polystyrene petri dish and was kept in an ambient condition for homogenization and subsequently for the gelation process for 28 days. The product was then dried in an oven at 40 °C for 7 days, afterwards a multistep drying process up to 120 °C was carried out. The dried samples were heated at 700 °C for 3 h with a heating rate of 2.5 °C/min to obtain a powder material. The powder was milled to a grain size of d50 = 2 μm.
Zein (Z3625) in form of powder was obtained from Sigma-Aldrich, Co. Anhydrous ethyl alcohol with 99.8 pct purity (POCH, Poland), glycerol (POCH, Poland) and purified water were used as components of the dispersing phase utilized for EPD.
To prepare the suspensions, a solution of ethyl alcohol, purified water and glycerin in appropriate concentrations, given in Table I, was stirred by a magnetic stirrer, (IKA model Ro 5, Germany). The appropriate amount of zein (Table I) was then weighed and gently added to the mixing solution. The OHAUS Corporation model PA214CM/1 scale (Switzerland) was used to weigh the suspension components. The mixture was stirred until the zein was dissolved and placed in a POLSONIC Sonic-3 ultrasonic bath (Poland), to break up any remaining zein agglomerates. The various portions of MSGG (Table I) were added to the prepared solution of zein and stirred using a magnetic stirrer for 10 min. The suspension was then ultrasonicated to crumble residual agglomerates of particles. The pH values of the suspensions were measured with an ELMETRON CPC-505 pH meter (Poland). All parameters of the suspension preparation and the deposition process are summarized in Table II.
Due to the lack of literature describing the preparation of a suspension for the EPD of MSGG/zein coatings, the knowledge describing the preparation of zein solution[32] and bioactive glasses (BG)/zein suspensions was used to elaborate preparation methods.[31,53] The chemical composition of the dispersing phase (90 vol pct ethanol, 10 vol pct water, 20 wt pct glycerol) was developed based on the knowledge of the EPD of BG/zein coatings on the stainless steel and magnesium substrates.[31,53] Zein content in the solution was investigated in our previous work[54] in relation to the adhesion of pure zein coatings to the titanium substrates. We have shown that the coatings deposited from a solution with a zein concentration of 200 g/dm3 had the highest adhesion strength. The selection of the MSGG concentration range (10–80 g/dm3) in our present work was based on the BG concentrations in suspensions used by Rehman (5 g/dm3)[53] and Meyer et al. (80 g/dm3).[31] The use of several concentrations of MSGG in suspensions was intended to determine its influence on the homogeneity and adhesion of coatings to the substrate. The conditions for the preparation of suspensions (stirring time and dispersion time) were selected experimentally on the basis of the period needed to minimize the presence of zein and MSGG agglomerates. The selection of the voltage range and deposition time (Table II) was made on the basis of the literature data on the EPD of BG‘s/zein coatings.[31,53]
A Malvern Zetasizer Nanodevice (Malvern Instruments Ltd., UK) equipped with a titrator was employed to investigate the zeta potential and conductivity of MSGG using the laser Doppler velocimetry (LDV) technique. A diluted suspension consisting of 0.4 g/dm3 MSGG, 2 g/dm3 zein, 0.24 g/dm3 glycerol, ethyl alcohol and distilled water in the proportion 9/1 was used for the measurements to allow the laser to work unhampered. Hydrochloric acid (HCl) and sodium hydroxide (NaOH) of various concentrations were used as titrants. The estimation of the potential values was based on the Debye–Hückel equation.
The EPD setting consisted of a two-electrode cell, spaced 10 mm apart, where the working electrode was a Ti-13Nb-13Zr alloy sample and 316L-SS plate was used as the counter electrode. To generate the desired direct current (DC) with constant voltage, an EX752M Multimode PSU power supply (AIM-TTI, UK), was used. A Tektronix DMM 4040 multimeter (TEKTRONIX, UK), was used to investigate the effect of the suspension’s chemical composition, voltage and time on current density throughout the process. A pulsed direct current (PDC) was generated by an Autolab PGSTAT 302 N (the Netherland) potentiostat/galvanostat, while deposition was also attempted in order to minimize the pore concentration in the coating volume.
To investigate the coating adhesion to the substrates, tape test in consonance with the ASTM D3359-17B standard was used. The tests consist of making two perpendicular cuts approx. 20–30 mm in length to form a grid using a multi-blade knife. Standardized tape was put to the grid area, then gently pressed with a pencil eraser and left for 60 s, then torn off at 90º. Then the grid surface was assessed on the substrates and the coating were classified according to the class adhesion included in the ASTM standard.
The information about the structure of the bioactive glass was received from the Fourier transform infrared spectroscopy (FTIR) investigation using a Vertex 70 V spectrometer (Bruker). Measurements were performed in the 400–4000 cm−1 wavenumber range with 128 scans and a resolution of 4 cm−1. The phase composition of the glass was analysed by X-ray diffractometry utilizing a diffractometer of a Philips X'Pert Pro, PANanalytical, UK operating at 30 kV and 30 mA with Cu-Kα radiation.
The specific surface areas (SBET) of the material were established by N2 adsorption employed the BET analysis (Nova 1200e, Quantachrome). A Nova NanoSEM450 (FEI, the Netherlands) scanning electron microscope (SEM) and a JEM-2010 ARP (JEOL, Japan) transmission electron microscope (TEM) were used to investigate the morphology and microstructure of the MSGG particles and coatings. The sample of MSGG for TEM investigation was prepared by putting a drop of MSGG in ethyl alcohol on a Cu grid covered with carbon and then drying it. The focus ion beam (FIB) milling utilizing a QUANTA 3D200i device, FEI, the Netherlands, was conducted for the specimen (lamella) preparation for the TEM study of the deposited layer. Selected area electron diffraction (SAED) patterns were used for phase identification. Fast Fourier transform (FFT) patterns and Inverse Fast Fourier transform (IFFT) images were created using Gatan’s ‘Digital Micrograph’ software. The volume fraction of MSGG in the coatings was investigated by peeling off coatings from the substrate to obtain films. The films were located in a ceramic crucible and heated at 500 °C for 60 min to completely degrade the zein. The weight of the crucible was checked before and after heating, and it was assumed that after heating only the inorganic component of the coating (MSGG) was present. Knowing the MSGG and zein density (2.46 and 1.23 g/cm3, respectively), the volume fraction of the components within the coatings was determined.
A WYKO NT930 optical profilometer (Veeco) was utilized to analyze the surface roughness of the substrate and coating. A Krüss DSA25E goniometer (Germany) was applied to determine the contact angle (CA) and surface free energy (SFE) of the materials. Total SFE, with its dispersive and polar components, was calculated using the Owens–Wendt–Rabel–Kaelble (OWRK) procedure.
All the electrochemical studies were performed in a Faraday’s cage and environment of Ringer’s electrolyte solution at pH equal to 7.4 in Ar atmosphere at a temperature of 37 °C. The electrolyte contained 8.6 g/dm3 NaCl, 0.3 g/dm3 KCl, 0.25 g/dm3 CaCl2 in water. The polarization measurements were considered in the electric potential range of − 1.5−2.2 V and for a rate of electric potential changes equivalent to 1 mV/s. The measurements were carried out using a classical cell equipped with three electrodes. The working electrode was an alloy substrate with or without coating. The counter electrode consisted of platinum wire. Potentials were measured vs. SCE (saturated calomel electrode). The measurements were conducted with an AUTOLAB PGSTAT302 potentiostat/galvanostat (the Netherlands). The corrosion current density (icorr) was determined by limiting the current density which moved through the passivating film, turning into a measure of the film’s protective performance.[55] During the EIS measurements, the amplitude was 10 mV and the frequency was in the range of 105–10−3 Hz. The EIS results were fitted using the ZView software. The errors were reduced utilizing the chi-squared criteria for fitting the experimental data of the EIS.
The bioactive properties of MSGG powder were evaluated by incubation in simulated body fluid (SBF), prepared following the procedure developed by Kokubo[56] at 37 °C for 1, 3, 7, and 14 days with the sample weight [g] to SBF volume [ml] ratio of 1:500. After washing with anhydrous ethyl alcohol and drying at RT the samples were investigated by FTIR. The changes in the concentration of Ca, P, and Si in SBF during incubation of the glasses were evaluated by inductively coupled plasma atomic emission spectrometry (ICP–OES; Plasma 40, Perkin Elmer). The measurements were repeated three times and the results were given as mean ± standard deviation (SD).
A concentrated version of SBF (1.5SBF) was used for the bioactivity test of the coated substrate, as explained below (Section III–F). The 1.5SBF solution was prepared according to Kokubo[57] and consisted of HCl (Tarchem, Poland), NaHCO3 (Chempur, Poland), Na2SO4 (Eurochem, Poland), KCl (Poch, Poland), K2HPO4 · 3H2O (Chempur, Poland), MgCl2 · 6H2O (Chempur, Poland), NaCl (Poch, Poland), NH2C(CH2OH)3 (Poch, Poland) and CaCl2 (Eurochem, Poland) dissolved in high-quality water (UHQ) (Chempur, Poland). The prepared solution had a pH of 7.4. The samples were then placed in 50 ml SBF in an INC 108 CO2 incubator (Memmert, Germany) at 36.5 °C ± 0.5 °C for 21 days. After this time, the samples were investigated using SEM and FTIR.
The antibacterial properties of zein and MSGG/zein coatings were further evaluated against gram-positive (S. aureus) and gram-negative (E. coli) bacteria by using Alamar blue assay. Briefly, the samples were sterilized under UV light for 1 h on each side, respectively, in a 24-multi-well plate and 1 ml of the bacteria suspension (OD at 600 nm = 0.001) was added in each well. The samples were incubated for 90 min in agitation (90 rpm) at 37 °C to allow the separation between adherent biofilm cells and non-adherent floating planktonic cells (separation phase). Subsequently, supernatants containing planktonic cells were discarded and replaced with 1 ml of fresh medium. Biofilm was grown at 37 °C for 24 h. After the incubation period, the samples were moved in new well-plates prior to evaluation. 1 ml of the alamarBlue® solution was added in each well and the samples were incubated in the dark for additional 5 h at 37 °C. After the incubation period 3 aliquots of 100 μl from each sample were transferred into 96-well plate and the OD values were read at a wavelength of 570 nm with a reference wavelength of 600 nm by micro-plate reader. Each coating type was analyzed in triplicate and pure zein coatings were used as a control. The reduction of Alamar blue reagent [ pct] was calculated according to the manufacturer’s protocol:
with \({E}_{oxi,570}\)= molar extinction coefficient of oxidized AlamarBlue at 570 nm = 80,586, \({E}_{oxi, 600}\)= molar extinction coefficient of oxidized AlamarBlue at 600 nm = 117,216, \({E}_{red, 570}\)= molar extinction coefficient of reduced AlamarBlue at 570 nm = 155,677, \({E}_{red, 600}\)= molar extinction coefficient of reduced AlamarBlue at 600 nm = 14,652, \({A}_{570}\)= absorbance of test wells at 570 nm. \({A}_{600}\)= absorbance of test wells at 600 nm, \({C}_{570}\)= absorbance of negative control well (media, AlamarBlue Reagent, no cells) at 570 nm, \({C}_{600}\)= absorbance of negative control well (media, AlamarBlue Reagent, no cells) at 600 nm.
SEM analysis was used to evaluate the bacterial attachment and spreading on the coatings. After culturing for 24 h, bacteria were fixed on the surface of the coatings using mixture solutions of glutaraldehyde, formaldehyde, sucrose and distilled water. Finally, the samples were dehydrated with increasing ethanol series (30, 50, 60, 70, 85 and 99.5 pct) for 10 min, respectively.
3 Results and Discussion
3.1 Mesoporous Sol–Gel Glass
The molar composition of the MSGG was as follows: 70 SiO2, 25 CaO, 5 P2O5. The sol–gel glass was ground, and the obtained powder subjected to a series of examinations aimed at determining particle size, chemical composition, specific surface area and pore distribution. The MSGG particles had an irregular shape with grain size in the range of 0.3 µm to 3.2 µm (Figure 2(a)). Measurements made using the laser diffractometer revealed that the average particle size of the glass particles was approx. 2.1 µm. The occurrence of Si, Ca, P and oxygen was validated by SEM–EDS microanalysis (Table III).
The FTIR spectrum of the glass sample showed the three main bands that were connected to characteristic oscillations of the Si–O–Si bond in the silicate network (Figure 2(b)). The first, sharp band at 468 cm−1 was attributed to the bending motion of the bridging oxygen atom in the Si–O–Si bond, the next band at 795 cm−1 corresponded to symmetric stretching of the bridging Si–O–Si bond between the SiO4 tetrahedra, while the third large band at 1067 cm−1 arose from the anti-symmetric stretching of the Si–O–Si group.[58] A weak band at 956 cm−1 represented the stretching oscillations of the non-bridging Si–O (Si–O-NBO) bond probably with one non-bridging oxygen per SiO4 tetrahedron.[59,60]
Additionally, in the bands at 468 cm−1 and 1067 cm−1, bending oscillations of O–P–O bonds and asymmetric stretching oscillations of O-P bonds in the PO4 tetrahedra were present, respectively. Moreover, the single peak that was present at 603 cm−1 pointed to phosphorus in the amorphous phase.[59] The shoulder at about 1190 cm−1 was attributed to the anti-symmetric stretching of the Si–O–Si bridging bonds. The bands at 1635 cm−1 and 3445 cm−1 represented bending and stretching oscillations of the –O–H groups in the water molecules absorbed in the samples, respectively. The half width and relatively high intensity of the 3445 cm−1 band also suggests the contribution of the stretching oscillations of O–H bonds arising from silanol (Si–OH) groups present in the glass structure.
The bioactive properties of the MSGG particles were assessed in relation to calcium phosphate-forming capability during immersion test in SBF. The formation of the CaP layer on the surface of the glass particles was examined by FTIR spectroscopy (Figure 2(b)). The result confirmed that new double bands in the range of 560 – 602 cm−1, assigned to the O–P–O bending mode, appeared after just 1 day of immersion in SBF. These bands are characteristic for crystalline calcium phosphate precipitations. A new characteristic band of CO32− bending vibrations at 873 cm−1 proved the crystallization of hydroxyapatite with carbonate substitution (CHA). The results of the ICP–OES analysis of SBF during different incubation times of MSGG particles are collected in Table IV.
The highest Ca concentration in SBF was observed at 1 day of incubation, and then Ca concentration was almost constant after the next time point. In contrast, the concentration of P after only 1 day of incubation was below the detection limit, which suggests the total adsorption of P from SBF to form calcium phosphate precipitations and thus limited further consumption of Ca ions. The release rate of Si from the glass particles to the fluid achieved the highest level after 1 day of incubation and was slightly reduced after 7/14 days of incubation. This behaviour arises from the formation of the CaP layer on the particles, which delayed the release of Si species. The particles exhibited a very high specific surface area of 318.5 ± 1.5 m2/g. The investigation of MSGG using XRD revealed an amorphous structure (Figure 2(c)). A broad peak at 2θ in the range of 20 to 35 deg was present in the pattern.
TEM investigations allowed to observe the mesoporous architecture of the sol–gel glass particles. It was found that about 95 pct volume fraction of particles contained highly ordered mesopores and the remaining particles contained non–ordered mesopores. As an example, Figure 3(a-c) shows particles with well–ordered mesopore channel structure and Figure 3(d) presents a particle with non–ordered mesopores. The particles contained typical 2D-hexagonal (p6mm symmetry) mesoporous channels (Figure 3(b)). The same symmetry of mesoporous channels was observed by Lopez-Noriega et al.[61] in highly ordered mesoporous SiO2-P2O5-CaO sol–gel glasses with a similar chemical composition. The images taken with the electron beam perpendicular to the channels (beam direction [110]) are shown in Figure 3(a,c), while the image in Figure 3(b) were taken with the beam parallel to the mesoporous channels (in [100] direction). Some of the particles contained regions with an ordered mesopore channel structure and regions with non–ordered mesopores. The size of particles observed in TEM was in the range of several dozen of nanometers to about 2 µm.
An electron diffraction investigation confirmed the XRD study and showed an amorphous atomic structure of the MSGG particles. A typical SAED pattern containing broad diffusion diffraction rings is shown in Figure 4(a). TEM-EDS microanalysis of the particles confirmed that the chemical composition was within the range determined using SEM–EDS (Table III), e.g. for the particle in Figure 4(a) following chemical composition was detected: (in at. pct) 63.1 O, 26.9 Si, 8.9 Ca and 1.1 P.
TEM image of the ordered MSGG particle recorded along the direction perpendicular to the channels and SAED pattern taken from the area marked by a circle (a). (b) Shows the FFT pattern of a square area of the particle in the TEM image, IFFT image and intensity profile (along the line in the IFFT image) based on which the pore size was determined
To measure the mesopore size, two-dimensional (2D) fast Fourier transform (FFT) of the digitized experimental TEM images was performed and inverse IFFT images were reconstructed from streaks present in the FFT patterns. The use of a mask around the streaks allowed a significant reduction of high-frequency non-periodic noise and to obtain stronger contrast images for pore size measurements. The pore size diameter was measured as a distance between the adjacent fringes in a line profile of the IFFT patterns of different particles with ordered mesopore channels. The pore size diameter of the particles was determined to be in the range of 3.2–8.1 nm. For example, the distance for adjacent fringes measured in a line profile in Figure 4(b) was 6.04 nm. It should be noted that the presence of such ordered mesopores with a small diameter in the glass particles provides a high surface area and volume of pores, much higher than those occurring in the glass with the same chemical composition produced by the conventional sol–gel method. Therefore, such particles can significantly contribute to enhancing bioactivity.[62]
3.2 Optimization of EPD Conditions and Adhesion Strength of the Coatings
Zein is a polymer that dissolves in hydrated alcohols. On the other hand, in literature[63] it was found that the ethyl alcohol/water ratio has a significant influence on the solubility of zein. The most optimal medium for zein solubility contains ethyl alcohol in the range of 60–90 pct,[64] but coatings deposited from a solution containing only alcohol and water were brittle and cracked. Research conducted by Xu et al.[65] has shown that the addition of glycerin has a positive effect on the plasticity of zein coatings. We showed previously,[54] that the ethyl alcohol and water proportion of 9/1 and 24 g/dm3 glycerol enabled the production of homogeneous and pure zein coatings on titanium with high adhesion strength. For this reason, in the present work, we used a previously developed zein solution to which MSGG particles in various contents of 10 g/dm3, 40 g/dm3 and 80 g/dm3 were added.
The stability of the suspensions was determined based on zeta potential values.[66] Another important factor responsible for successful coating deposition is conductivity,[67] as it can affect the stability of the particles or their mobility, which can lead to the inhibition of deposition.[68,69]
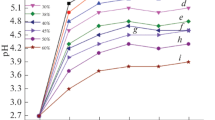
To perform correct measurements, a transparent suspension is needed, so that the light passing through it goes to the detector placed in front. For this purpose, in this work, the investigations were performed on a 100-fold dilution of the suspensions, which contains ethyl alcohol and distilled water in the ratio of 9/1, with the addition of 0.24 g/dm3 glycerol, 2 g/dm3 zein and various concentrations of MSGG (0.1, 0.2, and 0.4 g/dm3) to obtain reliable results. The zeta potential of the prepared suspensions for the original pH was, respectively: + 0.6 mV (10 g/dm3 MSGG) for pH 7.11, + 3.2 mV (40 g/dm3 MSGG) for pH 7.39 and + 3.5 mV (80 g/dm3 MSGG) for pH 7.66 (Figure 5). The results obtained for target suspensions demonstrated that the coatings would be obtained on the cathode at the pH range from 3 to 9. The conductivity of the solutions was the lowest near the starting pH of suspensions and maximal in acidic environments. Mishra et al.[70] indicated that the increase in conductivity is most likely due to an increase in free ions in the solution due to the addition of acids and bases to adjust the pH. These ions become major charge carriers, which reduces the mobility of the polymer chains and ceramic particles in the suspension.[71]
The literature does not describe the EPD mechanism of MSGG particles with zein. However, the EPD mechanism of bioglass (BG) with zein was reported previously by Meyer et al.[31] Authors claim that zein significantly influences the deposition process. Zein consists of different groups of amino acids, some negatively charged and some positively charged. Therefore, zein surrounds BG particles and, together with the deprotonated part of zein particles, binds to bioactive glass particles, which then migrate to the cathode. Similar mechanism may be active in the co-deposition of MSGG particles with zein. At acidic pH, MSGG particles as well as zein, investigated in our earlier work,[54] have positive values of zeta potential. It can therefore be assumed that MSGG particles surrounded by chains of zein migrate towards the negative electrode as a result of the applied electric field and settle on its surface forming a coating.
The MSGG/zein composite coatings were deposited from suspensions containing ethyl alcohol and distilled water in a ratio of 9/1, with the additives of 24 g/dm3 glycerol, 200 g/dm3 zein and various concentrations of MSGG, 10, 40 or 80 g/dm3 (Table I). Macroscopically uniform coatings were obtained at the voltage range of 3–10 V with a deposition time of 5 min. An attempt to apply a voltage below 3 V resulted in no coating deposition. In the case of higher voltage (above 10 V) the carbonization of the zein matrix on the surface of the substrate took place. When deposition time was shorter (3 and 4 min), the coatings were thin and nonuniformly distributed over the surface of the substrate. Longer deposition time (6 and 7 min) resulted in the appearance of folds and macroscopically visible pores on the coating. Open pores appeared on the surface of all coatings. The appearance of many pores on the coating's surface is likely the result of the electrolysis of water taking place during EPD.[72] The number of pores depended on the applied current voltage and the concentration of MSGG particles in suspensions. This phenomenon was more intense with increasing voltage and MSGG concentration in the suspensions (Figure 6).
The change in the weight of the coating as function of deposition time was determined. The mass of all coatings increased almost linearly as the process progressed. The weight gain was very small, amounting to thousandths of a gram (Figure 7(a)). The EPD rate was rapid at the beginning of the deposition process, reaching the highest value after about 60 s, and then decreased continuously to the end of the deposition (Figure 7(b)).
Pulse direct current EPD has been used to minimize the number of pores formed in the coating during EPD. Sufficiently short, pulsed DC (PDC) signals can prevent the formation of water electrolysis reaction products.[73,74,75] Several attempts were made to conduct pulse deposition with the present system by changing the cycle activity, pulse duration and time interval between pulses, even with dilute suspensions, but none has resulted in a homogeneous coating surface. For example, Figure 8(a) shows several coatings deposited with PDC EPD. Pores still appeared on the coating in a greater or similar number than when using DC EPD (Figure 8(b)).
The adhesion of coatings to the substrates was investigated by the tape tests. The test was performed for the coatings obtained using DC from suspensions with content of MSGG equal to 10 g/dm3, 40 g/dm3 and 80 g/dm3 (Figure 9). The coating adhesion classes were high and ranged from 4B (less than 5 pct of area removed) for the coatings obtained from suspension with 10 g/dm3 MSGG (Figure 9(a)) to 5B (0 pct of area removed) for coatings obtained from suspension contained 40 g/dm3 and 80 g/dm3 MSGG (Figure 9(b,c)). Composite coatings showed significantly higher adhesion to the substrates compared to pure zein coatings, which had an adhesion class to the substrate equal to 0B (the lowest adhesion class).
However, detailed SEM observations (Figure 9) showed delaminations of small coating areas close to the cuts. They were especially visible on coatings achieved from 10 and 80 g/dm3 MSGG suspensions (Figure 9(a,c) respectively).
3.3 Morphology and Microstructure of Coatings
Uniformly distributed glass particles and their agglomerates (Figure 10) with dimensions in the range of 1–8 µm and numerous pores (Figure 9) with diameter in the range of 30–50 µm were observed on coating surfaces achieved at a 5 V constant voltage for 5 min using suspensions of various MSGG contents. As the concentration of MSGG particles increased, numerous boundaries separating the agglomerates of MSGG particles could be noticed on the surface of the coatings. The boundaries presumably result from the shrinkage of the polymer while drying the coatings.
The volume fraction of MSGG particles in the coatings deposited from suspensions with various contents was determined as 4 ± 1 vol pct for 10 g/dm3, 6 ± 1 vol pct for 40 g/dm3 and 10 ± 1 vol pct for 80 g/dm3, respectively.
The microstructure of the coating showing the greatest adhesion to the substrate, e.g. achieved from the suspension with 40 g/dm3 MSGG, was examined by TEM on a sample prepared using FIB from the coating’s cross-section. The coating thickness in the investigated area varied from 6.1 µm to 9.4 µm (Figure 11(a)). The coating was dense, but pores with a diameter up to 300 nm derived from the EPD process or the lamella preparation using FIB were visible (Figure 11(b)). The single particles and agglomerates of the glass were evenly arranged in the zein matrix. The selected area electron diffraction patterns showed an amorphous structure of both MSGG and zein (Figure 11(b)). The proportion of MSGG particles in the zein matrix was lower near the substrate surface.
The overview of the investigated lamella observed by SEM with the marked thickness of the coating (a). Microstructure of the coating deposited from suspension 2 (Table I) at 5 V voltage and time of 5 min on the alloy and electron diffraction patterns of the zone marked with letter A and B for zein and MSGG, respectively (b), TEM
3.4 Influence of the Coating’s Surface Topography on Wetting Properties
The composite coatings had higher roughness compared to the titanium alloy substrate (Figure 12). The arithmetic average height (Ra) and root mean square roughness (Rq) of the coatings were at least 10 times higher than of the uncoated substrate (Table V). Meanwhile, the maximum height of the profile (Rmax) was at least 5 times higher for the coatings. Compared to the zein coating investigated in our previous study,[54] an increase in the values of roughness parameters was noticed. These differences were due to the presence of MSGG particles, their agglomerates and open pores. However, Ra and Rq decreased, while Rmax increased with rising MSGG volume fraction in the coating. A possible explanation for this phenomenon may be that, as the volume fraction of MSGG in the coating increased, the particles were increasingly densely packed, which led to a smoothing of the coating surface. Moreover, the open pores that appeared in the coating were deepened in coatings with higher volume fractions of MSGG.
The CA and the SFE were measured for the substrate material and coatings deposited at a constant 5 V voltage and a constant time of 5 min. In order to determine the SFE by the OWRK method, two liquids were used: polar water (H2O) and dispersive diiodomethane (CH2I2). All coatings decreased the CA of the alloy by around 20 deg (Table VI). The obtained CA was close to ideal wettability in the range of 35 to 80 deg for the early stage of protein attachment.[76]
All coatings showed higher SFE values (around 20 pct) than the substrate material. The results are similar to the data obtained in our previous work for a zein coating on titanium,[54] where the CA value was 43.5 ± 2.8 deg and the SFE was 59.5 ± 3.8 mN/m. It can be assumed that the MSGG particles were completely embedded in the zein matrix and therefore zein had a decisive effect on the coating surface properties. Dong et al.[77] found that zein has an amphiphilic character. Corradini et al.[78] reported that this phenomenon is due to occurrence in zein hydrophobic (proline, leucine, isoleucine) and hydrophilic (glutamine) proteins. Presumably, glutamine chains were responsible for the hydrophilic nature of the coatings, which might be presented on the top part of the coating. Based on the available literature,[79,80] regarding mesoporous glasses, it can be assumed that the mesoporosity of the glass used in this work will additionally decrease the contact angle of the substrate surface and rise its SFE during the zein degradation process.
3.5 Influence of Coatings on the Electrochemical Corrosion Resistance of the Alloy
The free corrosion potential was evaluated for a period of 12 h and results are presented in Figure 13(a). The value of this potential of the coated alloy was significantly higher than for the virgin alloy. The potential value of the coating at the beginning of the measurement was − 0.05 V and changed in time, constantly decreasing its value to the level of about − 0.19 V after approximately 2 h. Subsequently, the value of the OCP gradually increased and was characterized by some fluctuation through the next 5 h. Finally, after about 8 h, the OCP of the coated alloy achieved a constant value of − 0.12 V. The Eocp values for the virgin alloy showed a constant value equal to − 0.45 V. In the OCP measurement, but in general, in all chemical reactions related to the surface of a material, the morphological conditions and the chemical state of the substrate (initial and during the test) together with the chemico-physical parameters related to the solution (and to the whole measurement environment), play a significant role on the obtained result. In our studies, unstable OCP values during 7 h of exposure in Ringer’s solution can be strongly connected with the presence of open pores and biodegradable properties of the coatings. On the contrary, the excellent adhesion to the substrate achieved for the moderate porosity coatings (deposited from suspensions with 40 g/dm3 MSGG) substantially impeded corrosion processes. The OCP values of the coated alloy were significantly more positive than in the case of the virgin alloy. The results demonstrated that the coated substrate had enhanced corrosion resistance in comparison with the virgin (non-coated) sample.
The cyclic potentiodynamic polarization curves of the alloy with the MSGG/zein coating in Ringer’s solutions are presented in Figure 13(b). The potentiodynamic measurements confirmed the results of the stationary potential results, which indicated that the coated substrate material exhibited significantly better resistance to corrosive processes in Ringer’s electrolyte compared to the virgin one.
The polarization curves confirmed that the virgin titanium alloy (uncoated substrate) was easily passivated, which inhibited its dissolution.[81,82] A very wide plateau range was observed on both obtained curves: for the virgin substrate it equaled between − 0.30 and 1.60 V, and for the MSGG/zein coated substrate it was significantly longer in the potential range from − 1.04 to 1.60 V. The current densities for the anodic area was significantly higher for the virgin substrate compared with the coated one. On the contrary, in our previous study,[54] we showed that titanium with pure zein coating revealed insignificantly weaker corrosion resistance in Ringer’s electrolyte at the normal body temperature than the virgin titanium. This behavior may be due to several factors, for example: (i) lower thickness and (ii) lower adhesion strength of the zein coating in comparison with composite coatings, and (iii) the presence of glass particles in the composite coatings, which inhibited corrosion processes. The OCP and polarization results are presented in Table VII.
Figure 14 shows the Nyquist plots and equivalent circuit for the virgin substrate and the coated substrate immersed in Ringer’s electrolyte at 37 °C. For the virgin substrate, an experimental diagram of the equivalent circuit was built from electrolyte resistance (R1) in series with the parallel combination of a constant phase element (CPE) and a polarization resistance (R2).
In the present work, the experimental diagram of an equivalent circuit for the MSGG/zein coated alloy consisted of the electrolyte resistance (R1) and a CPE-1 in parallel with a resistance (R2). Two last components were related to the response of the barrier layer, which is attached in series with a constant phase element (CPE-2) associated with the diffusion process in the layer. The rough and porous MSGG/zein coating can cause a double-layer capacitance to appear as a constant phase element with a CPE-P value between 0.9 and 1. According to the parameters from the equivalent circuit shown in Table VIII, the CPE1-P value for MSGG/zein coated alloy equaled about 0.92. On the contrary, the CPE2-P value for coated alloy equaled almost 0.1. The constant phase element CPE2-P is the Warburg impedance due to mass transfer to the electrode surface, but in examined coatings these processes were taking place much slower than in typical Warburg impedance. Alike, Sreekanth et al.[83] performed the EIS studies of ZM21 magnesium alloy by developing a ceramic coating on its surface using the plasma electrolytic oxidation technique. It is worth underlining that the authors obtained a similar equivalent circuit where R2 corresponded to the pore resistance. The inner barrier layer resistance of the oxide film respectively, CPE1 and CPE2 represented constant phase elements corresponding to the pores and inner barrier layer. It was concluded that they obtained a layer containing low porosity which does not extend down to the substrate. Moreover, this outer layer helped in the development of a pore-free barrier layer beneath the outer porous layer with very high corrosion resistance.
3.6 In-Vitro Acellular Bioactivity of the MSGG/Zein Coated Alloy
Due to the fact that the bioactive MSGG particles are embedded in zein matrix, which does not allow their direct dissolution, and Ca solubility occurs only in particles located close to the surface, 1.5 SBF was selected for the immersion of the coated alloy in this study. 1.5SBF has higher ion concentrations, which gives a higher chance of formation of nucleation sites for apatite crystal growth and could finally lead to an homogeneous distribution of apatite crystals on the investigated material as shown by Taruta et al.[84]
The formation of a continuous and thick layer of calcium phosphate with cauliflower-like morphology was observed on the coating surface after 3 weeks of immersion in 1.5SBF (Figure 15). Microanalysis of the chemical composition of the formed layers showed the presence of Ca and P. The atomic Ca:P ratio was 1.5 ± 0.1. The characteristic pattern of bands in the range of 560–610 cm−1 (560 higher intensity, 605 lower intensity) and the remaining bands at 1040 cm−1 + 870/1420/1470 cm−1 and 1630/3400 cm−1, as well as the Ca/P ratio, indicate that the formed layer is a carbonate hydroxyapatite (HCA) structure.
3.7 Antibacterial Assay on MSGG/Zein Coatings
In order to investigate the influence of MSGG on the antibacterial performance of the samples, pure zein and MSGG/zein composite coatings prepared from suspensions with the highest and lowest MSGG concentration (10 g/dm3 and 80 g/dm3) were analyzed by Alamar blue assay. All MSGG-containing coatings showed antibacterial properties against gram-negative E. coli and gram-positive S. aureus in comparison to pure zein coatings (Figure 16(a)). Nevertheless, no significant differences in the antibacterial efficiency of the coatings with varying glass concentrations were found. According to the SEM observations, S. aureus and E. coli could attach and spread on the smooth surface of pure zein coatings as both bacteria types exhibited intact cell membranes (Figure 16(b)). In comparison, the number of adherent bacteria on the composite coatings reduced significantly, suggesting the antibacterial properties of MSGG. As reported in the literature, the rapid ion exchange between the alkaline ions of the bioactive glass and the hydrogen ions from the solution can create a hostile alkaline environment for bacteria.[85] Moreover, the increased osmolarity and osmotic pressure due to MSGG ion release can affect the bacterial morphology.[86]
4 Conclusions
-
1.
The cathodic electrophoretic deposition of macroscopically homogeneous MSGG/zein composite coatings was developed. The optimal composition of the suspensions was ethyl alcohol and distilled water in the ratio 9/1 with 200 g/dm3 of zein, 40 g/dm3 or 80 g/dm3 MSGG and 20 wt pct glycerol. Both DC EPD and PDC EPD led to obtaining coatings with open porosity. The open pore number was greater with increasing voltage and MSGG concentration in the suspensions.
-
2.
The coating microstructure consisted of MSGG particles embedded in the zein matrix. Increasing the content of MSGG particles in the EPD suspension resulted in an increase in their volume fraction in the coating.
-
3.
The coatings achieved from mixtures containing 40 g/dm3 and 80 g/dm3 of MSSG showed excellent adhesion strength (adhesion class 5B). The adhesion class of the coatings obtained from the mixture containing 10 g/dm3 of MSSG was slightly lower (4B).
-
4.
The coatings revealed high roughness, about 10 times higher compared to the uncoated substrate and high wettability with contact angle with water of 45º-49º, which are promising factors for promoting cell attachment and spreading.
-
5.
The MSGG/zein coated alloy exhibited increased resistance to corrosion in Ringer’s electrolyte compared to the virgin alloy and the alloy coated with pure zein.
-
6.
The formation of a continuous carbonate hydroxyapatite layer was observed on the coating surface after immersion in 1.5SBF, which indicates its bioactivity. MSGG/zein coatings showed superior antibacterial activity against gram-positive S. aureus and gram-negative E. coli bacterial strains in comparison to pure zein coatings.
The obtained results show great potential of the MSGG/zein coatings for biomedical application.
References
M. Kaur and K. Singh: Mater. Sci. Eng. C, 2019, vol. 102, pp. 844–62.
R. Bobbili and V. Madhu: Mater. Sci. Eng. A, 2017, vol. 700, pp. 82–91.
I. Cvijović-Alagić, Z. Cvijović, S. Mitrović, M. Rakin, D. Veljović, and M. Babić: Tribol. Lett., 2010, vol. 40, pp. 59–70.
H.Y. Hu, L. Zhang, Z.Y. He, Y.H. Jiang, and J. Tan: Mater. Sci. Eng. C, 2019, vol. 98, pp. 279–87.
P. Kumar, G.S. Mahobia, S. Mandal, V. Singh, and K. Chattopadhyay: Corros. Sci., 2021, vol. 189, 109597.
M. Geetha, A.K. Singh, K. Muraleedharan, A.K. Gogia, and R. Asokamani: J. Alloys Compd., 2001, vol. 329, pp. 264–71.
L.T. Duarte, S.R. Biaggio, R.C. Rocha-Filho, and N. Bocchi: Corros. Sci., 2013, vol. 72, pp. 35–40.
C. Marker, S.L. Shang, J.C. Zhao, and Z.K. Liu: Comput. Mater. Sci., 2018, vol. 142, pp. 215–26.
L. Zhou, T. Yuan, R. Li, J. Tang, M. Wang, and F. Mei: J. Alloys Compd., 2018, vol. 762, pp. 289–300.
M. Morinaga: A Quantum Approach to Alloy Des., 2019, pp. 77–94.
P. Majumdar, S.B. Singh, and M. Chakraborty: J. Mech. Behav. Biomed. Mater., 2011, vol. 4, pp. 1132–44.
M. Niinomi and M. Nakai: Int. J. Biomater., 2011, 836587.
M. Geetha, U. Kamachi Mudali, A.K. Gogia, R. Asokamani, and B. Raj: Corros. Sci., 2004, vol. 46, pp. 877–92.
M. Geetha, A.K. Singh, A.K. Gogia, and R. Asokamani: J. Alloys Compd., 2004, vol. 384, pp. 131–44.
J. Raphel, M. Holodniy, S.B. Goodman, and S.C. Heilshorn: Biomaterials, 2016, vol. 84, pp. 301–14.
D.D. Bosshardt, V. Chappuis, and D. Buser: Periodontol 2000, 2017, vol. 73, pp. 22–40.
D.V. Nazarov, V.M. Smirnov, E.G. Zemtsova, N.M. Yudintceva, M.A. Shevtsov, and R.Z. Valiev: ACS Biomater. Sci. Eng., 2018, vol. 4, pp. 3268–81.
C.E. Tanase, M. Golozar, S.M. Best, and R.A. Brooks: Colloids Surf. B Biointerfaces, 2019, vol. 176, pp. 176–84.
D. Zhitomirsky, J.A. Roether, A.R. Boccaccini, I. Zhitomirsky, M. Science, and M.S. West: O. Ls, 2008, vol. 9, pp. 1853–60.
R. Paliwal and S. Palakurthi: J. Control. Release, 2014, vol. 189, pp. 108–22.
Y. Luo and Q. Wang: J. Appl. Polym. Sci., 2014, vol. 131, pp. 1–2.
S. Gong, H. Wang, Q. Sun, S.T. Xue, and J.Y. Wang: Biomaterials, 2006, vol. 27, pp. 3793–99.
Y. Yuan, M. Ma, Y. Xu, and D. Wang: Trends Food Sci. Technol., 2022, vol. 120, pp. 1–5.
T. Miyoshi, K. Toyohara, and H. Minematsu: Polym. Int., 2005, vol. 54, pp. 1187–90.
X. Zhang, C. Dong, Y. Hu, M. Gao, and G. Luan: Food Sci. Hum. Wellness, 2021, vol. 10, pp. 270–77.
L. Feng, J. Zhu, G. Wang, Y. Tang, H. Chen, W. Jin, F. Wang, B. Mei, Z. Xu, and R. Song: Plant Mol. Biol., 2009, vol. 69, pp. 649–59.
N. Matsushima, G.I. Danno, H. Takezawa, and Y. Izumi: Biochim. Biophys. Acta Protein Struct. Mol. Enzymol., 1997, vol. 1339, pp. 14–22.
H. Tan, H. Zhou, T. Guo, J. Li, C. Zhang, S. Wang, Y. Zhang, and L. Ma: Food Chem., 2022, vol. 374, 131563.
J.W. Lawton: Cereal Chem., 2002, vol. 79, pp. 1–8.
Y. Zhang, L. Cui, X. Che, H. Zhang, N. Shi, C. Li, Y. Chen, and W. Kong: J. Control. Release, 2015, vol. 206, pp. 206–19.
N. Meyer, L. Rivera, T. Ellis, J. Qi, M. Ryan, and A. Boccaccini: Coatings, 2018, vol. 8, p. 27.
S. Kaya and A.R. Boccaccini: J. Coatings Technol. Res., 2017, vol. 14, pp. 683–89.
Y. Ahmed and M.A. Ur Rehman: Surf. Interfaces, 2020, vol. 20, p. 100589.
P. Zhou, Y. Xia, X. Cheng, P. Wang, Y. Xie, and S. Xu: Biomaterials, 2014, vol. 35, pp. 10033–45.
L. Ramos-Rivera, J. Dippel, and A.R. Boccaccini: ECS Trans., 2018, vol. 82, pp. 73–80.
D. Arcos and M. Vallet-Regí: Acta Biomater., 2010, vol. 6, pp. 2874–88.
A. Balamurugan, G. Sockalingum, J. Michel, J. Fauré, V. Banchet, L. Wortham, S. Bouthors, D. Laurent-Maquin, and G. Balossier: Mater. Lett., 2006, vol. 60, pp. 3752–57.
S. Vermeulen, Z.T. Birgani, and P. Habibovic: Biomaterials, 2022, vol. 283, 121431.
A. Kumar, C.R. Mariappan, and B.S. Sarahan: J. Non. Cryst. Solids, 2019, vol. 505, pp. 431–37.
F. Baino, S. Fiorilli, and C. Vitale-Brovarone: Bioengineering, 2017, vol. 4, pp. 1–8.
J. Xiao, Q. Wei, J. Xue, Z. Liu, Z. Li, Z. Zhou, F. Chen, and F. Zhao: Colloids Surf. A Physicochem. Eng. Asp., 2022, vol. 642, p. 128693.
X. Yan, X. Huang, C. Yu, H. Deng, Y. Wang, Z. Zhang, S. Qiao, G. Lu, and D. Zhao: Biomaterials, 2006, vol. 27, pp. 3396–403.
F. Foroutan, B.A. Kyffin, I. Abrahams, A. Corrias, P. Gupta, E. Velliou, J.C. Knowles, and D. Carta: ACS Biomater. Sci. Eng., 2020, vol. 6, pp. 1428–37.
L. Ramos-Rivera, A. Cochis, S. Biser, E. Canciani, S. Ferraris, L. Rimondini, and A.R. Boccaccini: Bioact. Mater., 2021, vol. 6, pp. 1479–90.
M.R. Zakaria, H. Md Akil, M.H. AbdulKudus, F. Ullah, F. Javed, and N. Nosbi: Compos. Part B Eng., 2019, vol. 176, p. 107313.
S. Hu, W. Li, H. Finklea, and X. Liu: Adv. Colloid Interface Sci., 2020, vol. 276, 102102.
Y. Ma, J. Han, M. Wang, X. Chen, and S. Jia: J. Mater., 2018, vol. 4, pp. 108–20.
S. Obregón, G. Amor, and A. Vázquez: Adv. Colloid Interface Sci., 2019, vol. 269, pp. 236–55.
R. Sikkema, K. Baker, and I. Zhitomirsky: Adv. Colloid Interface Sci., 2020, vol. 284, 102272.
B. Hoomehr, K. Raeissi, F. Ashrafizadeh, S. Labbaf, and M. Kharaziha: Ceram. Int., 2021, vol. 47, pp. 34959–69.
Ö. Varlık, Y. Göncü, and N. Ay: Mater. Chem. Phys., 2022, vol. 282, p. 125927.
O. Gerard, A. Numan, S. Krishnan, and M. Khalid: J. Energy Storage, 2022, vol. 50, 104283.
M.A. Ur Rehman: Prosthesis, 2020, vol. 2, pp. 211–24.
F. Maciąg, T. Moskalewicz, K. Kowalski, A. Łukaszczyk, Z. Hadzhieva, and A.R. Boccaccini: Materials (Basel), 2021, vol. 14, pp. 1–24.
E. McCafferty and G.K. Hubler: J. Electrochem. Soc., 1978, vol. 125, pp. 1892–93.
T. Kokubo and H. Takadama: Biomaterials, 2006, vol. 27, pp. 2907–15.
T. Kokubo, S. Ito, Z.T. Huang, T. Hayashi, S. Sakka, T. Kitsugi, and T. Yamamuro: J. Biomed. Mater. Res., 1990, vol. 24, pp. 331–43.
A.G. Kalampounias: Bull. Mater. Sci., 2011, vol. 34, pp. 299–303.
I. Lebecq, F. Désanglois, A. Leriche, and C. Follet-Houttemane: J. Biomed. Mater. Res. A, 2007, vol. 83, pp. 156–68.
H. Aguiar, J. Serra, P. González, and B. León: J. Non. Cryst. Solids, 2009, vol. 355, pp. 475–80.
A. López-Noriega, D. Arcos, I. Izquierdo-Barba, Y. Sakamoto, O. Terasaki, and M. Vallet-Regí: Chem. Mater., 2006, vol. 18, pp. 3137–44.
I. Izquierdo-Barba and M. Vallet-Regi: Biomed. Glas., 2015, vol. 1, pp. 140–50.
K. Hu, X. Huang, Y. Gao, X. Huang, H. Xiao, and D.J. McClements: Food Chem., 2015, vol. 182, pp. 275–81.
Y. Chen, Z. Zhao, G. Xia, F. Xue, C. Chen, and Y. Zhang: Int. J. Biol. Macromol., 2020, vol. 146, pp. 179–92.
H. Xu, Y. Chai, and G. Zhang: J. Agric. Food Chem., 2012, vol. 60, pp. 10075–81.
S.A. Siti, S.H. Amirnordin, H.A. Rahman, H.Z. Abdullah, and H. Taib: Adv. Mater. Res., 2013, vol. 795, pp. 334–37.
A. Molaei and M.A. Yousefpour: Prot. Met. Phys. Chem. Surfaces, 2019, vol. 55, pp. 302–09.
B. Ferrari and R. Moreno: J. Eur. Ceram. Soc., 2010, vol. 30, pp. 1069–78.
B. Ferrari and R. Moreno: J. Eur. Ceram. Soc., 1997, vol. 17, pp. 549–56.
M. Mishra, Y. Sakka, T. Uchikoshi, and L. Besra: J. Ceram. Soc. Japan, 2013, vol. 121, pp. 348–54.
L. Besra and M. Liu: Prog. Mater. Sci., 2007, vol. 52, pp. 1–61.
L. Besra, T. Uchikoshi, T.S. Suzuki, and Y. Sakka: J. Am. Ceram. Soc., 2008, vol. 91, pp. 3154–59.
D.J. Barrett, M.D. Linley, S.M. Best, and R.E. Cameron: Biofabrication, 2019, vol. 11, p. 045017.
L. Besra, T. Uchikoshi, T.S. Suzuki, and Y. Sakka: J. Eur. Ceram. Soc., 2009, vol. 29, pp. 1837–45.
B. Neirinck, O. Van Der Biest, and J. Vleugels: J. Phys. Chem. B, 2013, vol. 117, pp. 1516–26.
G. Khang, J.M. Rhee, J.H. Lee, I. Lee, and H.B. Lee: Korea. Polym. J., 2000, vol. 8, pp. 276–84.
F. Dong, G.W. Padua, and Y. Wang: Soft Matter, 2013, vol. 9, pp. 5933–41.
E. Corradini, P.S. Curti, A.B. Meniqueti, A.F. Martins, A.F. Rubira, and E.C. Muniz: Int. J. Mol. Sci., 2014, vol. 15, pp. 22438–70.
K. Huang, S. Cai, G. Xu, M. Ren, X. Wang, R. Zhang, S. Niu, and H. Zhao: Surf. Coatings Technol., 2014, vol. 240, pp. 137–44.
K. Huang, S. Cai, G. Xu, X. Ye, Y. Dou, M. Ren, and X. Wanga: J. Alloys Compd., 2013, vol. 580, pp. 290–97.
M.F. López, A. Gutiérrez, and J.A. Jiménez: Surf. Sci., 2001, vol. 482–485, pp. 300–05.
M. Metikoš-Huković, A. Kwokal, and J. Piljac: Biomaterials, 2003, vol. 24, pp. 3765–75.
D. Sreekanth, N. Rameshbabu, K. Venkateswarlu, C. Subrahmanyam, L. Rama Krishna, and K. Prasad Rao: Surf. Coat. Technol., 2013, vol. 222, pp. 31–37.
S. Taruta, K. Kidokoro, T. Yamakami, T. Yamaguchi, K. Kitajima, M. Endo, and N. Saito: J. Nanomater., 2011, vol. 2011, pp. 1–9.
S. Kaya, M. Cresswell, and A.R. Boccaccini: Mater. Sci. Eng. C, 2018, vol. 83, pp. 99–107.
F. Kurtuldu, N. Mutlu, M. Michálek, K. Zheng, M. Masar, L. Liverani, S. Chen, D. Galusek, and A.R. Boccaccini: Mater. Sci. Eng. C, 2021, vol. 124, p. 112050.
Acknowledgments
This work was supported by the National Science Centre, Poland (Decision No. DEC-2018/31/G/ST5/00429) and by the German Science Foundation (DFG) (Project BO 1191/25-1). The authors appreciate a valuable contribution of Prof. A. Kopia to XRD investigation, Dr Ł. Cieniek, Dr A. Fiołek to SEM investigation (AGH-University of Science and Technology) as well as Dr M. Gajewska (ACMIN) for FIB lamella preparation.
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Maciąg, F., Moskalewicz, T., Cholewa-Kowalska, K. et al. Development and Investigation of Mesoporous Bioactive Glass/Zein Coatings Electrodeposited on Titanium Alloy for Biomedical Applications. Metall Mater Trans A 54, 241–260 (2023). https://doi.org/10.1007/s11661-022-06864-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11661-022-06864-2