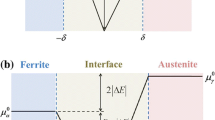
Abstract
Eutectoid transformation in cast irons may proceed in the stable or the metastable systems giving ferrite and graphite for the former and pearlite for the latter. The present work demonstrates that composition profiles across ferrite/pearlite boundaries are smooth and similar to those issued from the solidification step. No trace of long-range diffusion of substitutional solutes due to austenite decomposition could be observed. In turn, this ascertains that both stable and metastable transformations proceed with the product matrix—either ferrite or pearlite—inheriting the parent austenite content in substitutional solutes. This result sustains a physical model for eutectoid transformation based on the so-called local para-equilibrium which is commonly used for describing solid-state transformation in steels.












Similar content being viewed by others
References
J.M. Schissler: Hommes et Fonderie, 1986, April, pp. 13-23.
J.M. Schissler: Hommes et Fonderie, 1986, pp. 13–23.
K.B. Rundman: Proc. of 101 Casting Congress, 1997, AFS, paper 97-117.
J. Lacaze: ASM Handbook, Cast Irons, vol. 1A, 2016 (to appear)
[5] J. Lacaze and J. Sertucha: Inter. J. Cast Met. Res., 2016, vol. 29, pp. 74-78.
[6] U. Ekpoom and R.W. Heine: AFS Trans., 1978, vol. 86, pp. 281-286.
[7] R. Boeri and F. Weinberg: Cast Metals, 1993, vol. 6, pp. 153-158.
[8] J. Lacaze, C. Wilson and C. Bak: Scand. J. Metall., 1994, vol. 23, pp. 151-163.
[9] V. Gerval and J. Lacaze: ISIJ International, 2000, vol. 40, pp. 386-392.
[10] A. Hultgren: Trans. ASM, 1947, vol. 39, pp. 915-1005
D. Venugopalan: Proceedings of the International Symposium on Fundamentals and applications of ternary diffusion, G.R. Purdy, ed., Hamilton, Canada, 1990, pp. 173–83.
[12] X. Guo and D.M. Stefanescu: IJCMR, 1999, vol. 11, pp. 437-441.
[13] J. Lacaze, A. Boudot, V. Gerval, D. Oquab and H. Santos, Metall. Mater. Trans. 28A (1997) 2015-2025
[14] W.C. Johnson and B.V. Kovacs: Metall. Trans. A, 1978, vol. 9, 219-229.
[15] Z. Bofan and E.W. Langer: Scand. J. Metall., 1984, vol. 13, pp. 23-31.
[16] B.V. Kovacs: AFS Trans., 1980, vol. 89, 79-96.
V. Gerval and J. Lacaze: Proceedings of SP97, 4th Decennial International Conference on Solidification Processing, eds J. Beech and H. Jones, University of Sheffield, 1997, pp. 506–10.
[18] Benyan Pei, B. Björkman, B. Sundman and B. Jansson: CALPHAD, 1995, vol. 19, pp. 1-15.
[19] J. Miettinen: CALPHAD, 1998, vol. 22, pp. 231-256.
[20] J. Lacaze and B. Sundman: Metall. Trans. A, 1991, vol. 22, pp. 2211-2223.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted March 10, 2016.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendix
Appendix
The thermodynamic evaluation of the Fe-Sb phase diagram by Benyan Pei et al.[18] has been introduced in the available SSOL databank that was last updated in 1998. The only change made to this latter bank is the improvement of the Fe-C-Si system performed by Miettinen[19] to the earlier assessment by Lacaze and Sundman.[20] Owing to the very limited amount of antimony relevant for cast irons production (0.1 mass pct at most), it could be considered that appropriate calculations for Fe-C-Si-Sb alloys could be performed without further modifications. Figures A1 and A2 compare isopleth Fe-C sections of, respectively, the stable and metastable systems at 2.0 mass pct Si and 0 and 1 mass pct Sb. According to these figures, 1 mass pct antimony shifts slightly both the stable and metastable eutectoid ranges to higher temperature. The T α and T p reference temperatures used in the main text are shown in Figures A1 and A2, respectively.
Isopleth Fe-C section of the metastable Fe-C-Si-Sb phase diagram at 2.0 mass pct Si and 0 and 1 mass pct Sb in the temperature range of the eutectoid transformation. The T p reference temperature is assumed to be on the extrapolation of the γ/α equilibrium line as indicated with an arrow for the alloy at 1 mass pct Sb
Rights and permissions
About this article
Cite this article
Freulon, A., de Parseval, P., Josse, C. et al. Study of the Eutectoid Transformation in Nodular Cast Irons in Relation to Solidification Microsegregation. Metall Mater Trans A 47, 5362–5371 (2016). https://doi.org/10.1007/s11661-016-3692-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11661-016-3692-3