Abstract
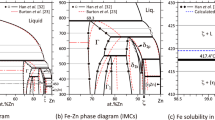
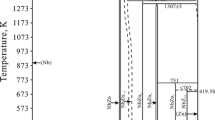
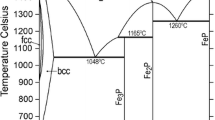
The thermodynamic parameters of the ZrO2-FeO-Fe2O3 system were assessed based on experimental data for the ZrO-FeO and ZrO2-Fe3O4 systems for the first time. The solubility of FeO and Fe2O3 in the ZrO2-based solid solutions and the solubility of ZrO2 in the Fe2O3 and Fe3O4 phases were taken into account and described by compound energy formalism. A partially ionic liquid model was used to describe the liquid phase. The isothermal section and liquidus surface of the ZrO2-FeO-Fe2O3 system were calculated. Data on binary systems were combined with the description of the ZrO2-FeO-Fe2O3 system. Phase diagrams were calculated using a thermodynamic description based on advanced models. An equilibrium between the metallic liquid and solid ZrO2 was calculated and compared with experimental data. Substantial differences between the calculations and the results of experiments were found, as in the calculations of previous research.






Similar content being viewed by others
References
S.V. Bechta, E.V. Krushilov, V.I. Almjashev, S.A. Vitol, L.P. Mezentsev, Y.B. Petrov, D.B. Lopuch, V.B. Khabensky, M. Barrachin, S. Hellmann, K. Froment, M. Fischer, W. Tromm, D. Bottomley, F. Defoort and V.V. Gusarov: J. Nucl. Mater., 2006, vol. 348, pp. 114-21.
V. Raghavan: Phase Diagrams of ternary Iron alloys, 1989, vol. 5, pp. 374-9.
H. Biermann, U. Martin, A. Aneziris, A. Kolbe, A. Müller, W. Schärfl and M. Herrman: Adv. Eng. Mat., 2009, vol. 11, pp. 1000-6.
W.A. Fischer and A. Hoffmann: Arch. Eisenhuttenwes, 1957, vol. 28, pp. 739-43.
S.V. Beshta, E.V. Krushilov, V.I. Almjashev, S.A. Vitol, L.P. Mezentsev, Y.B. Petrov, D.B. Lopukh, V.B. Khabenskii, M. Barrachin, S. Hellmann and V.V. Gusarov: Rus. J. Inorg. Chem., 2006, vol. 51, pp. 325-31.
T.S. Jones, S. Kimura and A. Muan: J. Amer. Ceram. Soc., 1967, vol. 50, pp. 137-42.
R.H.G.A. Kiminami: Ceramica, 1987, vol. 33(213), pp. 207–09.
R.H.G.A. Kiminami: Ceramica, 1988, vol. 34(213), pp. 121–23.
T. Katsura, M. Wakihara, S.I. Hara and T. Sugihara: J. Solid State Chem., 1975, vol. 13, pp. 107-13.
Y.B. Petrov, Y.P. Udalov, J. Slovak and Y.G. Morozov: Glass Phys. Chem., 2002, vol. 28, pp. 139-46.
R.J. Fruehan: Metall. Trans., 1974, vol. 5, pp. 345–47.
D. Janke and W.A. Fischer: Arch. Eisenhuttenwes, 1976, vol. 47, pp. 195-8.
W. Huang: CALPHAD, 2004, vol. 28, pp. 153-7.
P.-Y. Chevalier and E. Fischer: Unpublished Research, 2003, cited by Landolt-Bernstein, Thermodynamic Properties of Inorganic Materials compiled by SGTE, Group IV (Physical Chemistry), Binary systems—subvolume B, Binary systems from Cs-K to Mg-Zr, Springer, Berlin, 2005.
L. Kjellquist, M. Selleby and B. Sundman: CALPHAD, 2008, vol. 32, pp. 577-92.
C. Wang: unpublished research, 2006.
C. Wang, M. Zinkevich and F. Aldinger: J. Am. Ceram. Soc., 2006, vol. 89, pp. 3751-8.
I.-H. Jung, S.A. Decterov and A.D. Pelton: Metall. Mater. Trans. B, 2004, vol. 35B, pp. 493-507.
M. Hillert and M. Selleby: Scand. J. Metall., 1990, vol. 19, pp. 23-5.
H.L. Lukas, S.G. Fries and B. Sundman: Computational thermodynamics. The CALPHAD method, Cambridge University Press, Cambridge, 2007.
Acknowledgments
The authors would like to thank the German Research Foundation (DFG) for its financial support of the Collaborative Research Center Trip-Matrix Composites (SFB799).
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted August 5, 2014.
Rights and permissions
About this article
Cite this article
Fabrichnaya, O., Pavlyuchkov, D. Assessment of Experimental Data and Thermodynamic Modeling in the Zr-Fe-O System. Metall Mater Trans A 47, 152–159 (2016). https://doi.org/10.1007/s11661-015-2805-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11661-015-2805-8