Abstract
Summary
We developed a new model for predicting bone mineral density on chest radiographs and externally validated it using images captured at facilities other than the development environment. The model performed well and showed potential for clinical use.
Purpose
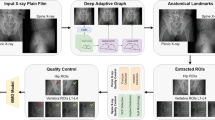
In this study, we performed external validation (EV) of a developed deep learning model for predicting bone mineral density (BMD) of femoral neck on chest radiographs to verify the usefulness of this model in clinical practice.
Methods
This study included patients who visited any of the collaborating facilities from 2010 to 2020 and underwent chest radiography and dual-energy X-ray absorptiometry (DXA) at the femoral neck in the year before and after their visit. A total of 50,114 chest radiographs were obtained, and BMD was measured using DXA. We developed the model with 47,150 images from 17 facilities and performed EV with 2914 images from three other facilities (EV dataset). We trained the deep learning model via ensemble learning based on chest radiographs, age, and sex to predict BMD using regression. The outcomes were the correlation of the predicted BMD and measured BMD with diagnoses of osteoporosis and osteopenia using the T-score estimated from the predicted BMD.
Results
The mean BMD was 0.64±0.14 g/cm2 in the EV dataset. The BMD predicted by the model averaged 0.61±0.08 g/cm2, with a correlation coefficient of 0.68 (p<0.01) when compared with the BMD measured using DXA. The accuracy, sensitivity, and specificity of the model were 79.0%, 96.6%, and 34.1% for T-score < -1 and 79.7%, 77.1%, and 80.4% for T-score ≤ -2.5, respectively.
Conclusion
Our model, which was externally validated using data obtained at facilities other than the development environment, predicted BMD of femoral neck on chest radiographs. The model performed well and showed potential for clinical use.




Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author, YT, upon reasonable request.
References
Salari N, Ghasemi H, Mohammadi L, Behzadi MH, Rabieenia E, Shohaimi S, Mohammadi M (2021) The global prevalence of osteoporosis in the world: a comprehensive systematic review and meta-analysis. J Orthop Surg Res 16:609. https://doi.org/10.1186/s13018-021-02772-0
Wade SW, Strader C, Fitzpatrick LA, Anthony MS, O'Malley CD (2014) Estimating prevalence of osteoporosis: examples from industrialized countries. Arch Osteoporos 9:182. https://doi.org/10.1007/s11657-014-0182-3
Barrett-Connor E (1995) The economic and human costs of osteoporotic fracture. Am J Med 98:3S–8S. https://doi.org/10.1016/s0002-9343(05)80037-3
Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A (2007) Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res 22:465–475. https://doi.org/10.1359/jbmr.061113
Johnell O (1997) The socioeconomic burden of fractures: today and in the 21st century. Am J Med 103:20S–25S; discussion 25S-26S. https://doi.org/10.1016/s0002-9343(97)90023-1
Rachner TD, Khosla S, Hofbauer LC (2011) Osteoporosis: now and the future. Lancet 377:1276–1287. https://doi.org/10.1016/S0140-6736(10)62349-5
World Health Organization (2023) WHO criteria for diagnosis of osteoporosis. 4BoneHealth. http://www.4bonehealth.org/education/world-health-organization-criteria-diagnosis-osteoporosis/2023.5.8. Accessed 10 Aug 2023
Bolotin HH, Sievänen H (2001) Inaccuracies inherent in dual-energy X-ray absorptiometry in vivo bone mineral density can seriously mislead diagnostic/prognostic interpretations of patient-specific bone fragility. J Bone Miner Res 16:799–805. https://doi.org/10.1359/jbmr.2001.16.5.799
Mueller D, Gandjour A (2009) Cost-effectiveness of using clinical risk factors with and without DXA for osteoporosis screening in postmenopausal women. Value Health 12:1106–1117. https://doi.org/10.1111/j.1524-4733.2009.00577.x
Jang M, Kim M, Bae SJ, Lee SH, Koh JM, Kim N (2022) Opportunistic osteoporosis screening using chest radiographs with deep learning: development and external validation with a cohort dataset. J Bone Miner Res 37:369–377. https://doi.org/10.1002/jbmr.4477
Yamamoto N, Sukegawa S, Kitamura A, Goto R, Noda T, Nakano K, Takabatake K, Kawai H, Nagatsuka H, Kawasaki K, Furuki Y, Ozaki T (2020) Deep learning for osteoporosis classification using hip radiographs and patient clinical covariates. Biomolecules 10:1534. https://doi.org/10.3390/biom10111534
Widyaningrum R, Sela EI, Pulungan R, Septiarini A (2023) Automatic segmentation of periapical radiograph using color histogram and machine learning for osteoporosis detection. Int J Dent 2023:6662911. https://doi.org/10.1155/2023/6662911
Zhang B, Yu K, Ning Z, Wang K, Dong Y, Liu X, Liu S, Wang J, Zhu C, Yu Q, Duan Y, Lv S, Zhang X, Chen Y, Wang X, Shen J, Peng J, Chen Q, Zhang Y et al (2021) Corrigendum to “Deep learning of lumbar spine X-ray for osteopenia and osteoporosis screening: a multicenter retrospective cohort study” [Bone 140, November 2020, 115561]. Bone 153:116143. https://doi.org/10.1016/j.bone.2021.116143
Wang F, Zheng K, Lu L, Xiao J, Wu M, Kuo CF, Miao S (2023) Lumbar bone mineral density estimation from chest X-ray images: anatomy-aware attentive multi-ROI modeling. IEEE Trans Med Imaging 42:257–267. https://doi.org/10.1109/TMI.2022.3209648
Hsieh CI, Zheng K, Lin C, Mei L, Lu L, Li W, Chen FP, Wang Y, Zhou X, Wang F, Xie G, Xiao J, Miao S, Kuo CF (2021) Automated bone mineral density prediction and fracture risk assessment using plain radiographs via deep learning. Nat Commun 12:5472. https://doi.org/10.1038/s41467-021-25779-x
Sato Y, Yamamoto N, Inagaki N, Iesaki Y, Asamoto T, Suzuki T, Takahara S (2022) Deep learning for bone mineral density and T-score prediction from chest X-rays: a multicenter study. Biomedicines 10:2323. https://doi.org/10.3390/biomedicines10092323
Adams M, Chen W, Holcdorf D, McCusker MW, Howe PD, Gaillard F (2019) Computer vs human: deep learning versus perceptual training for the detection of neck of femur fractures. J Med Imaging Radiat Oncol 63:27–32. https://doi.org/10.1111/1754-9485.12828
Altman DG, Vergouwe Y, Royston P, Moons KG (2009) Prognosis and prognostic research: validating a prognostic model. BMJ 338:b605. https://doi.org/10.1136/bmj.b605
Chlap P, Min H, Vandenberg N, Dowling J et al (2021) A review of medical image data augmentation techniques for deep learning applications. J Med Imaging Radiat Oncol 65(5):545–563. https://doi.org/10.1111/1754-9485.13261 Epub 2021 Jun 19
Tank VH, Ghosh R, Gupta V, Sheth N, Gordon S, He W, Modica SF, Prestigiacomo CJ, Gandhi CD (2016) Drug eluting stents versus bare metal stents for the treatment of extracranial vertebral artery disease: a meta-analysis. J Neurointerv Surg 8:770–774. https://doi.org/10.1136/neurintsurg-2015-011697
Kanda Y (2013) Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant 48:452–458. https://doi.org/10.1038/bmt.2012.244
Mounach A, Abayi DA, Ghazi M et al (2009) Discordance between hip and spine bone mineral density measurement using DXA: prevalence and risk factors. Semin Arthritis Rheum 38(6):467–471. https://doi.org/10.1016/j.semarthrit.2008.04.001 Epub 2008 Jun 24
Lips P, van Schoor NM (2005) Quality of life in patients with osteoporosis. Osteoporos Int 16:447–455. https://doi.org/10.1007/s00198-004-1762-7)
Lorentzon M (2019) Treating osteoporosis to prevent fractures: current concepts and future developments. J Intern Med 285(4):381–394. https://doi.org/10.1111/joim.12873. Epub 2019 Jan 18 https://onlinelibrary.wiley.com/journal/13652796
Ramspek CL, Jager KJ, Dekker FW, Zoccali C, van Diepen M (2021) External validation of prognostic models: what, why, how, when and where. Clin Kidney J 14:49–58. https://doi.org/10.1093/ckj/sfaa188
Collins GS, Moons KGM (2019) Reporting of artificial intelligence prediction models. Lancet 393:1577–1579. https://doi.org/10.1016/S0140-6736(19)30037-6
Snell KIE, Archer L, Ensor J, Bonnett LJ, Debray TPA, Phillips B, Collins GS, Riley RD (2021) External validation of clinical prediction models: simulation-based sample size calculations were more reliable than rules-of-thumb. J Clin Epidemiol 135:79–89. https://doi.org/10.1016/j.jclinepi.2021.02.011
Mukaka MM (2012) Statistics corner: a guide to appropriate use of correlation coefficient in medical research. Malawi Med J 24:69–71
Cetin A, Ertürk H, Celiker R et al (2001) The role of quantitative ultrasound in predicting osteoporosis defined by dual X-ray bsorptiometry. Rheumatol Int 20(2):55–59. https://doi.org/10.1007/pl00006857
Clowes JA, Peel NFA, Eastell R (2006) Device-specific thresholds to diagnose osteoporosis at the proximal femur: an approach to interpreting peripheral bone measurements in clinical practice. Osteoporos Int 17:1293–1302. https://doi.org/10.1007/s00198-006-0122-1
Gemalmaz A, Discigil G, Sensoy N et al (2007) Identifying osteoporosis in a primary care setting with quantitative ultrasound: relationship to anthropometric and lifestyle factors. J Bone Miner Metab 25(3):184–192. https://doi.org/10.1007/s00774-006-0741-9 Epub 2007 Apr 20
Trimpou P, Bosaeus I, Bengtsson BA et al (2010) High correlation between quantitative ultrasound and DXA during 7 years of follow-up. Eur J Radiol 73(2):360–364. https://doi.org/10.1016/j.ejrad.2008.11.024 Epub 2009 Jan 8. https://www.sciencedirect.com/science/article/pii/S0720048X08006554
Oral A, Esmaeilzadeh S, Yalıman A et al (2019) The ability of calcaneal and multisite quantitative ultrasound variables in the identification of osteoporosis in women and men. Turk J Phys Med Rehabil 65(3):203–215. https://doi.org/10.5606/tftrd.2019.1894 PMID: 31663068; PMCID: PMC6797920. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6797920/
Chanprasertpinyo W, Punsawad C, Khwanchuea R et al (2023) Comparison between calcaneus quantitative ultrasound and the gold standard DXA in the ability to detect osteoporosis in chronic obstructive pulmonary disease patients. J Orthop Surg Res 18(1):778. https://doi.org/10.1186/s13018-023-04211-8 Erratum in: J Orthop Surg Res. 2023 Nov 15; 18(1):868. PMID: 37845656; PMCID: PMC10577968
Jones T, Davie MW (1998) Bone mineral density at distal forearm can identify patients with osteoporosis at spine or femoral neck. Br J Rheumatol 37(5):539–543. https://doi.org/10.1093/rheumatology/37.5.539https://academic.oup.com/rheumatology/article/37/5/539/1783093?login=true
Azami A, Anari H, Iranparvar M et al (2019) Comparison of bone mineral densitometry at 2 sites versus 3 sites in patients suspicious for osteoporosis. Clin Med Insights Arthritis Musculoskelet Disord:12. https://doi.org/10.1177/1179544119849017
Yue C, Ding N, Xu LL et al (2022) Prescreening for osteoporosis with forearm bone densitometry in health examination population. BMC Musculoskelet Disord 23(1):377. https://doi.org/10.1186/s12891-022-05325-6 PMID: 35459140; PMCID: PMC9027342
Adami S, Zamberlan N, Gatti D et al (1996) Computed radiographic absorptiometry and morphometry in the assessment of postmenopausal bone loss. Osteoporos Int 6(1):8–13. https://doi.org/10.1007/BF01626531
Dey A, McCloskey EV, Taube T et al (2000) Metacarpal morphometry using a semi-automated technique in the assessment of osteoporosis and vertebral fracture risk. Osteoporos Int 11(11):953–958. https://doi.org/10.1007/s001980070034.B4:B32https://link.springer.com/article/10.1007/s001980070034
Hyldstrup L, Nielsen SP (2001) Metacarpal index by digital X-ray radiogrammetry: normative reference values and comparison with dual X-ray absorptiometry. J Clin Densitom 4(4):299–306. https://doi.org/10.1385/jcd:4:4:299https://www.sciencedirect.com/science/article/pii/S1094695006603624
Rosholm A, Hyldstrup L, Backsgaard L et al (2001) Estimation of bone mineral density by digital X-ray radiogrammetry: theoretical background and clinical testing. Osteoporos Int 12(11):961–969. https://doi.org/10.1007/s001980170026
Boonen S, Nijs J, Borghs H et al (2005) Identifying postmenopausal women with osteoporosis by calcaneal ultrasound, metacarpal digital X-ray radiogrammetry and phalangeal radiographic absorptiometry: a comparative study. Osteoporos Int 16(1):93–100. https://doi.org/10.1007/s00198-004-1660-z Epub 2004 Jun 10
Lydick E, Cook K, Turpin J, Melton M, Stine R, Byrnes C (1998) Development and validation of a simple questionnaire to facilitate identification of women likely to have low bone density. Am J Manag Care 4:37–48
Cadarette SM, Jaglal SB, Kreiger N, McIsaac WJ, Darlington GA, Tu JV (2000) Development and validation of the Osteoporosis Risk Assessment Instrument to facilitate selection of women for bone densitometry. CMAJ 162:1289–1294
Cadarette SM, Jaglal SB, Murray TM, McIsaac WJ, Joseph L, Brown JP (2001) Evaluation of decision rules for referring women for bone densitometry by dual-energy X-ray absorptiometry. JAMA 286:57–63. https://doi.org/10.1001/jama.286.1.57
Acknowledgements
The authors express their sincere gratitude to the following healthcare facilities for their invaluable support and cooperation throughout this study: Eniwa Hospital, Japan Community Healthcare Organization Tokyo Shinjuku Medical Center, Kobayakawa Orthopaedic Rheumatology Clinic, Japanese Red Cross Aichi Medical Center Nagoya Daiichi Hospital, Nagoya Medical Center, Gamagori City Hospital, Fujita Health University Hospital, National Center for Geriatrics and Gerontology, Gifu Prefectural Tajimi Hospital, Municipal Ena Hospital, Mie University School of Medicine Nagai Hospital, Yokkaichi Municipal Hospital, Sanda City Hospital, Mitoyo General Hospital, Japan Community Healthcare Organization Ritsurin Hospital, and Naruo Orthopaedic Surgery Hospital. This study would not have been possible without their assistance and collaboration, and all authors are deeply grateful for their contribution to the success of this study. Additionally, the authors would like to extend their appreciation to Yusuke Iesaki, Masahiro Kiyono, Takashi Iwakura, Tetsujiro Ohno, Masahiro Hasegawa, Naoaki Osada, Tomonori Kobayakawa, Yasumori Sobue, Nobuyuki Okui, Shogo Tabata, Terufumi Kokabu, Yuichiro Abe, and Nobuyuki Fujita for their valuable contributions.
Funding
This work was supported by the Japanese Orthopaedic Association [grant numbers 2022-3].
Author information
Authors and Affiliations
Contributions
Takamune Asamoto, Yoichi Sato, and Yasuhiko Takegami conducted the experiments and drafted the manuscript. Takamune Asamoto prepared Figs. 1–4 and Table 1. Yasuhiko Takegami, Mitsuru Saito, and Shiro Imagama contributed to the study design and drafted the manuscript. Yoichi Sato, Shunsuke Takahara, Norio Yamamoto, Naoya Inagaki, and Satoshi Maki recruited the participants and collected the data. Yoichi Sato, Takamune Asamoto, and Yasuhiko Takegami analyzed the data. All authors have read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval
The study protocol was approved by Gamagori City Hospital’s Human Research Ethics Committee and Institutional Review Board. The study procedures were conducted in accordance with the principles embodied in the Declaration of Helsinki.
Consent for publication
Before publishing the research findings, the authors obtained consent to publication from all participants who agreed to have their data included in the study.
Conflicts of interest
None.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Asamoto, T., Takegami, Y., Sato, Y. et al. External validation of a deep learning model for predicting bone mineral density on chest radiographs. Arch Osteoporos 19, 15 (2024). https://doi.org/10.1007/s11657-024-01372-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11657-024-01372-9